AATS 2022 Annual Meeting: Boston
Hynes Convention Center and Sheraton Boston Hotel
May 14-17, 2022
Learn how we are transforming the future of thoracic surgery at booth 1409
Stop by our booth to:
- Experience our trusted portfolio and latest technologies
- Ask questions and engage with our team of product experts
- Meet 1-on-1 with Medtronic leadership
Join our live event at AATS 2022

Innovations in Thoracic Surgery
A panel discussion with Michael Ebright, M.D., David Shersher, M.D., and Paula Antonia Ugalde Figueroa, M.D. on the latest innovations in thoracic surgery: from single anesthetic event diagnosis and staging to advanced surgical interventions and cutting-edge technique.
Saturday, May 14, 7:00 p.m.
Featured at AATS 2022, Booth 1409: Medtronic Lung Health Solutions Product Portfolio
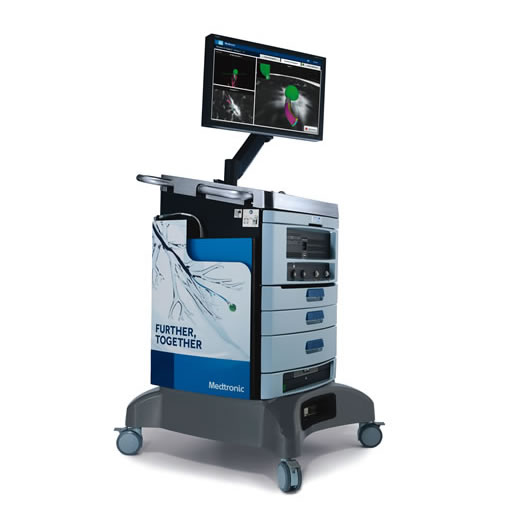
ILLUMISITE™ Platform
Correct for CT-to-body divergence, even in smaller nodules. Better visualize smaller lung lesions.1-5,† Biopsy with greater confidence.6 It’s all possible with our fluoroscopic navigation technology, continuous guidance, and innovative tools.
LungGPS™ Patient Management Platform
Designed to assist clinicians in identifying incidental nodules and monitoring lung nodule patients, manage workflows, and streamline thoracic tumor board collaboration.
EleVision™ IR Platform
The EleVision™ IR platform is a compact visualization system/platform that features native high-definition IR fluorescence and allows real-time fusion of visible and fluorescence imaging. It’s the only visualization system capable of real-time fluorescence signal intensity measurements in both open and laparoscopic procedures.‡

TipVision™ videoscope and EleVision™ 4K+ System
Achieve and maintain high OR workflow efficiency7,§ with the EleVision™ 4K+ platform and TipVision™ videoscope — innovative technology for world class visualization.
Meet the team
Get in touch with a Medtronic product expert or your local account manager to find the right solutions for you, your patients, and your facility.
† Not evaluated in lesions <10mm.
‡ Compared to Stryker SPY™* Elite Fluorescence imaging system, Stryker PinPoint™* Endoscopic fluorescence imaging, Stryker SPY-PHI™* SPY Portable Handheld Imaging System, Storz NIR/ICG Near™* Infrared Fluorescence Imaging, Olympus Visera™* EliteII, Stryker AIM 1588, Stryer AIM 1688. March 2020.
§ During Elevision HD2 usability lab, 32 out of 32 surgeons and nurses easily completed all pre-, intra-, and post-operative usability tasks prior to any training. Industrial design and user experience of the EleVision™ 4K system are equivalent to the EleVision™ HD 2 platform
1. Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology. 2019. doi:10.1111/ resp.13609.
2. Bhadra K, Mattingley J, Pritchett M. Electromagnetic navigation bronchoscopy with advanced fluoroscopy-based localization and intraprocedural local registration for the evaluation of peripheral pulmonary nodules. Paper presented at: CHEST Annual Meeting; Oct. 23, 2019; New Orleans, LA.
3. Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic navigation bronchoscopy with tomosynthesis-based visualization and positional correction: three-dimensional accuracy as confirmed by cone-beam computed tomography. J Bronchol Interv Pulmonol. 2021;28:10–20. doi: 10.1097/LBR.0000000000000687
4. Avasarala SK, Roller L, Katsis J, et al. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-Time Target Acquisition. Respiration. 2021; doi: 10 .1159 /000518009.
5. Dunn BK, Blaj M, Bowling M, et al. Evaluation of Electromagnetic Navigational Bronchoscopy Using Tomosynthesis-Assisted Visualization, Intraprocedural Positional Correction and Continuous Guidance for Evaluation of Peripheral Pulmonary Nodules. J Bronchology Interv Pulmonol. Published online Mar 10, 2022. doi:10.1097/LBR.0000000000000839.
6. Based on internal report #DGR00596, A summative survey results report; 16 physicians surveyed for the ILLUMISITE™ platform. March 2019.
7. Based on internal test report #RE00246399, EleVision™ HD 2 platform summative usability validation lab. February 2019.Data on file
The ILLUMISITE™ platform is indicated for displaying images of the tracheobronchial tree to aid the physician in guiding endoscopic tools or catheters in the pulmonary tract and to enable marker placement within soft lung tissue. It does not make a diagnosis and is not an endoscopic tool. Not for pediatric use.
WARNING: The ILLUMISITE™ platform may only be used by a qualified bronchoscopist.
CONTRAINDICATIONS: Flexible bronchoscopy should be performed only when the relative benefits outweigh the risks.
Absolute contraindications include, but are not limited to: lack of adequate facilities and personnel to care for emergencies such as cardiopulmonary arrest, pneumothorax, or bleeding, inability to adequately oxygenate the patient during the procedure.
Conditions that are usually considered absolute contraindications, unless risk-benefit assessment warrants the procedure: Coagulopathy or bleeding diathesis that cannot be corrected, severe obstructive airways disease, severe refractory hypoxemia, unstable hemodynamic status including dysrhythmias. Relative contraindications or conditions involving increased risk include but are not limited to: Recent myocardial infarction or unstable angina, uremia and pulmonary hypertension, and known or suspected pregnancy. Please refer to Instructions for Use for complete contraindication and risk information.
The LungGPS™ patient management platform is only available for sale in the United States.
The LungGPS™ patient management platform is an information management software only and does not diagnose or treat lung cancer nor replace lung cancer assessment in standard clinical practice. LungGPS™ should be used in conjunction with professional guidelines for patient management decision.
Contraindications: LungGPS™ is intended to integrate with specific file output of sequencing instruments such as Illumina sequencers as well as integrate with specific electronic medical records and external database warehouses. The system should not be used with any instruments that have not been tested and verified as compatible with LungGPS™.
©2022 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 04/2022–US-RE-2200224
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic