You inspired us to advance surgical energy.
Next-level performance1,†,‡ from your most trusted2 vessel sealing device
Introducing the LigaSure™ XP Maryland jaw sealer/divider, an evolution in vessel sealing inspired by your 25 million procedural experiences2 with our trusted LigaSure™ technology — and counting.
Gain the confidence to reliably seal thick tissue3,4,§,Ω with key performance enhancements:
- Longer seal plate and optimal jaw force for grasping, manipulating, and sealing thick tissue4,5,§,††
- Jaws that more securely grasp tissue3,6,‡‡
- 95% probability of seals that withstand burst pressures >360 mmHg7,††
An elevated level of device precision1,†,‡
- Curved, tapered jaws enable fine dissection and skeletonization of vessels3,Ω
- Provides sealing5,§§ and more precise cutting3,ΩΩ at the tip of the jaws
- Jaw design makes it easier to access tissue planes or create windows3,‡‡,ΩΩ
Improved procedural efficiency1,†,††† that turns
your focus to patients
- Continuous 360° rotation for undisrupted movement3,Ω,‡‡ and precise maneuverability3,Ω
- Longer jaws, seal plate, and cut length increase efficiency by sealing and transecting more tissue per bite3,‡‡,‡‡‡
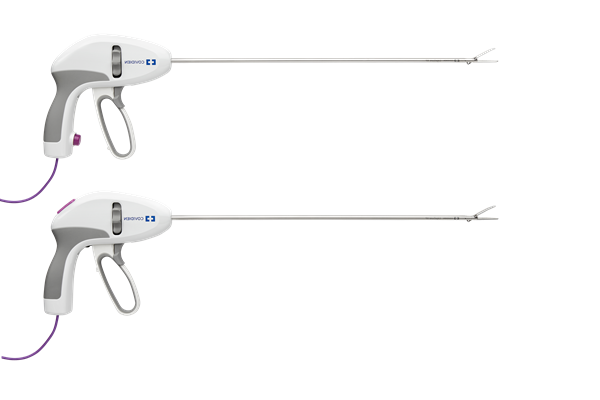
Confident performance. Refined to your needs.1,†,‡
Choose between one-step sealing or latching handle designs based on your procedural needs:
- One-step sealing minimizes the number of steps required to seal and transect tissue3,§§§
- Latching handle provides distinct and separate steps to grasp, seal, and cut3,ΩΩΩ
†The LigaSure™ XP Maryland jaw device is indicated for use in general surgery and such surgical specialties as colorectal, bariatric, urologic, vascular, thoracic, and gynecologic.
‡Compared to their current preferred device; 21 of 23 surgeons agreed during clinical procedures.
§Thick tissue is defined as nondissected vascular tissue or fatty tissue.
Ω29 out of 29 surgeons agree.
††Bench tissue may not be indicative of clinical tissue performance
‡‡Compared to legacy LigaSure™ devices.
§§30 out of 30 surgeons agree.
ΩΩ24 out of 29 surgeons agree.
†††Compared to their current preferred device; 20 of 23 surgeons agreed during clinical procedures.
‡‡‡28 out of 29 surgeons agree.
§§§15 out of 15 surgeons agree.
ΩΩΩ14 out of 14 surgeons agree.
1. Based on internal report #RE00457416 Rev A, Product introduction report: LigaSure™ XP Maryland jaw sealer/divider. May 2023.
2. Based on COGNOS and historical sales data, FY01–FY20. October 2019.
3. Based on internal report #RE00376094 Rev A, LigaSure™ XP Maryland jaw sealer/divider surgeon validation marketing report. Dec. 7–9 and 14–16, 2021.
4. Based on internal report #RE00442444 Rev A, Comparison of the renal artery bench bundle burst pressure performance with the LigaSure™ XP Maryland jaw sealer/divider, Voyant™* Maryland Fusion, Enseal™* X1 curved jaw, and LigaSure™ LF19XX devices. Jan. 23, 2023.
5. Based on internal report #RE00372649 Rev A, Archer surgeon summative evaluation report. March 22, 2022.
6. Based on internal report #RE00415591 Rev. A, LIG-48 LMXJXXX Archer BOE — jaw seal plate texture grip strength.
7. Based on internal report #RE00370099 Rev A, Verification of LigaSure™ XP Maryland jaw sealer/divider in a bench burst study burst test. March 30, 2022.
©2023 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. ™* Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. US-SE-2300145
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic