LiquiBand® XL skin closure system
Keep patients covered.
Get safe, secure, and effective closure for large and high-tension wounds1,2,†,‡ with LiquiBand® XL.

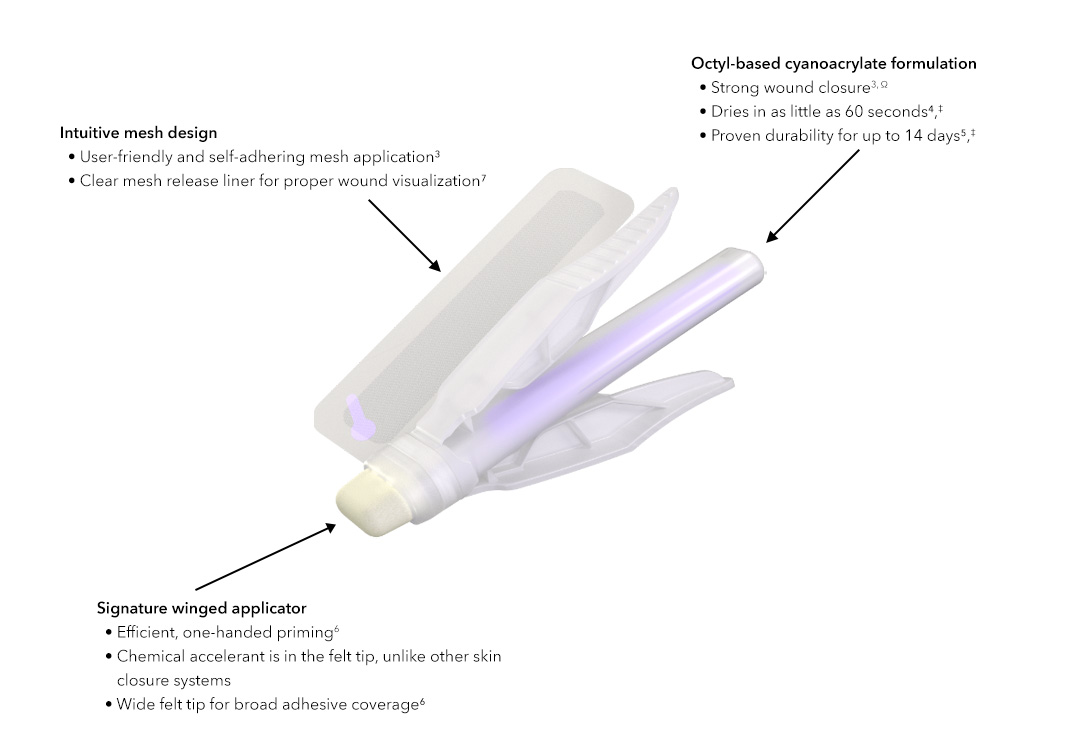
User-friendly application3
| Microbial protection during healing | ||
|---|---|---|
| Organism | Day 3 | Day 7 |
| Candida albicans | 100% | 100% |
| Staph. aureus | 100% | 100% |
| Escherichia coli | 100% | 100% |
| Staph. aureus (MRSA) | 100% | 100% |
| Pseudomonas aeruginosa | 100% | 100% |
| Enterobacter cloacae | 100% | 100% |
| Staph. Epidermidis | 100% | 100% |
| Aspergillus brasiliensis | 100% | 100% |
Microbial Protection
during healing
LiquiBand® XL provides an effective microbial barrier against gram positive, gram negative, and fungal microbes for at least 7 days.8,9,§
Strength you expect
The Liquiband® XL skin closure system provides equivalent would closure strength compared to Dermabond® Prineo® and exofin fusion®10-13,§
Equivalent wound closure strength than Dermabond® Prineo® and exofin fusion®12–14,§§

Additional resources


LiquiBand® XL skin closure system should be used in conjunction with, but not in place of, deep dermal stitches. Adverse effects include but not limited to infection, acute inflammation include erythema, edema, and drainage, wound dehiscence, bleeding, skin edge necrosis. Please refer to IFU for complete contraindication and risk information.
†Tension should be mitigated prior to application according to product IFU.
‡Based on AMS pre-clinical data, may not be indicative of clinical performance.
§Based on AMS bench testing data, may not be indicative of clinical performance.
ΩBased on AMS bench test data comparing to other adhesive, may not be indicative of clinical performance.
††Based on AMS in vitro testing, may not be indicative of clinical performance.
§§ Compared to Dermabond® Prineo®. Compared to exofin fusion®.Based on AMS bench testing data, may not be indicative of clinical performance.
ΩΩCompared to Dermabond® Prineo®. Compared to exofin fusion®.Testing was performed on the mesh only. Based on AMS bench test data comparing to other adhesive, may not be indicative of clinical performance.
1. Pre-Clinical Safety & Performance Eval Porcine Model 14 Days Section 16.1, 16.2 and 12.3. May 2021.
2. Based on AMS report #005-156. LiquiBand® XL Clinical Evaluation Report. Section 10. February 2021.
3. Based on AMS internal report #005-141: Internal & External Usability Study Report. Section 1.2 and Section 9.1. May 2021.
4. Pre-Clinical Safety & Performance Eval Porcine Model 14 Days, Section 15.6.1 and Table 11. May 2021.
5. Pre-Clinical Safety & Performance Eval Porcine Model 14 Days, Section 16.3.3. May 2021.
6. Based on AMS report #005-141: Internal & External Usability Study Report Section 11. May 2021.
7. Based on AMS report #005-150. Pre-Clinical Safety & Performance Eval Porcine Model 14 Days, Section 16.3.4. May 2021.
8. Based on AMS report #004-956. Microbial Barrier Summary Report LiquiBand® XL Zero Time, Section 10. January 2021.
9. Based on AMS report #005-166. Microbial Barrier Summary Report LiquiBand® XL 16 Weeks, Section 9.1 and Section 10.1. May 2021.
10. Based on AMS report # 005-137 Liquiband® XL & Competitor Bench Testing, Section 10.6. May 2021.
11.Based on AMS report # 005-121 Liquiband® XL Equivalence Report, Section 7.5. May 2021.
12.Based on AMS report #005-224: Project Yealm Competitive Testing Summary Report, Section 2.2.3 and Table 3. August 2021.
13.Based on AMS report #005-132: ASTM 2458-05 Wound Closure Bench Testing Report, Section 13.1. August 2021.
14.Based on AMS report #005-224: Project Yealm Competitive Testing Summary Report, Section 2.2.2 and Table 3. August 2021.
15.Based on AMS report# 005-134 Liquiband® XL & Competitor Bench Testing, Section 10.1. May 2021.
16.Based on AMS report # 005-861, Section 9.1, 9.2 and 10. September 2022.
17. LiquiBand® XL skin closure system #K211878 [510k]. Advanced Medical Solutions. August 2022.
18. Based on Dermabond® Prineo® discussion guide and exofin fusion® 510K submission (K191461).
©2023 Medtronic. Medtronic, Medtronic logo and Engineering the extraordinary are trademarks of Medtronic. LiquiBand® is a registered trademark of Advanced Medical Solutions Ltd. ™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. 02/2023 - US-WC-2200138 [WF#7540790]
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic