SGNA 52nd Annual Course
June 1–3, 2025 | Pittsburgh, PA
Visit us at booth #306
There is no time for compromise.
Empowering patient safety. Anytime, anywhere.
Stop by booth #306 to:
- Experience our capnography portfolio firsthand
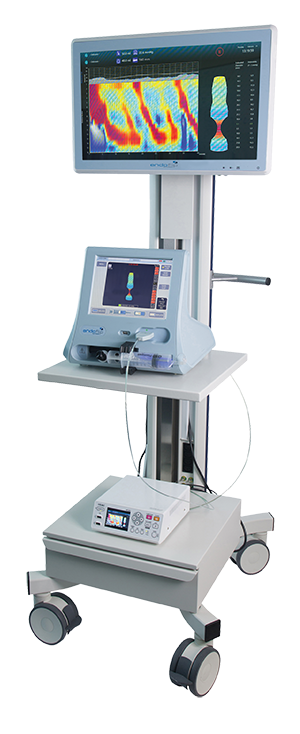
- Learn about the all-new RespArray™ patient monitor
- Discover how the Medtronic endoscopy portfolio supports proactive GI care
Join us in the Exhibit Hall Theater Learning Sessions:
"Improve workflow and safety with Microstream™ capnography"
Sunday, June 1 | 4:15–4:30 p.m. EDT
Speaker:
Brian D. Berry Jr., RN, BSN, CRNA, MS, MBA
Chief Nurse Anesthetist
Director, Perioperative Services
Independence Health System
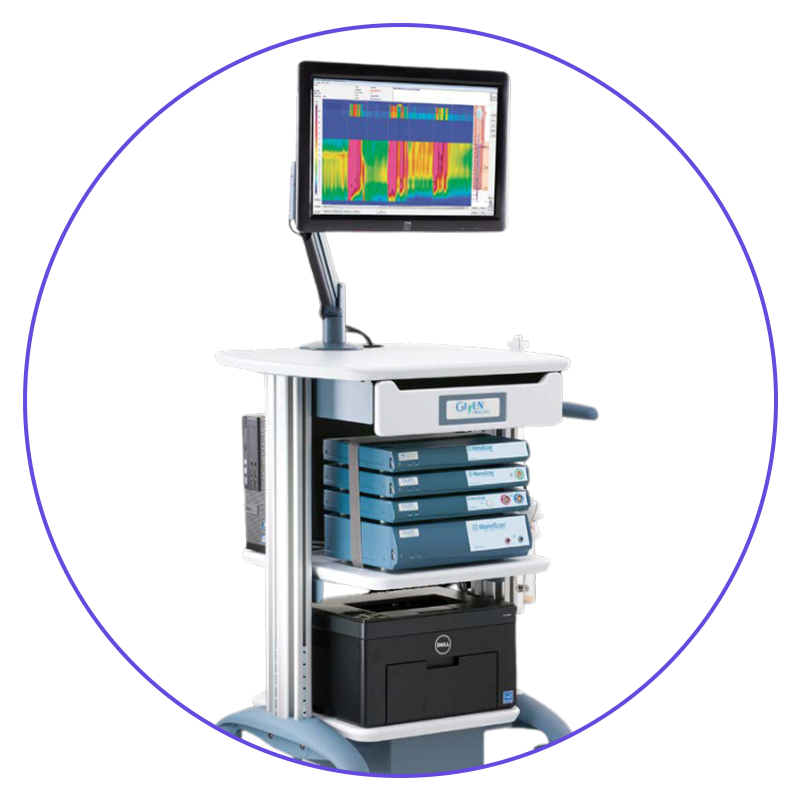
"Chicago Classification v4 Software Updates for ManoScan™ ESO high resolution manometry system"
Monday, June 2 | 12–12:30 p.m. EDT
Speaker:
Amber Powell
Senior Principal Global Market Development Manager
Esophageal & Gastric Disease
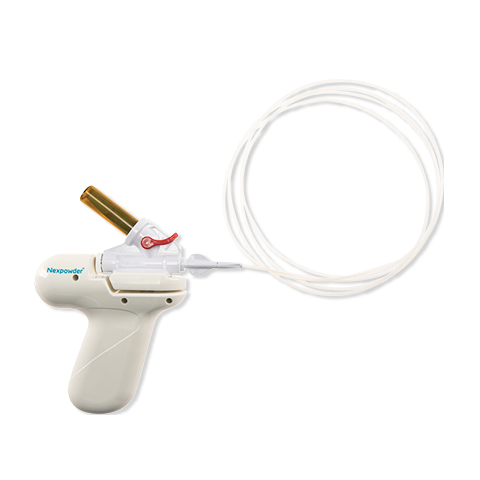
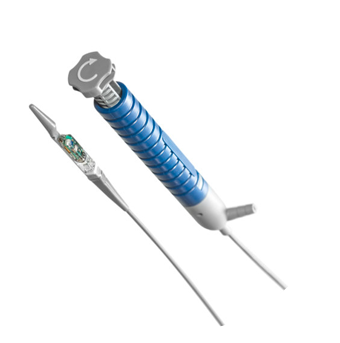
"Nexpowder™* endoscopic hemostasis system: A novel hemostatic agent for managing GI bleeding across the GI tract: Tips & Considerations"
Monday, June 2 | 12:30–1 p.m. EDT
Speaker:
Ross Delomel
Senior Global Market Development Manager
Endoscopic Therapeutics
Medtronic products featured at SGNA 2025:
Acute Care & Monitoring
Respiratory management
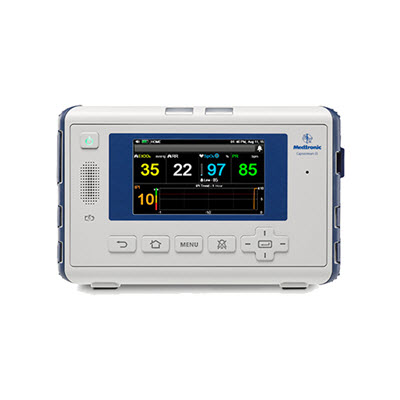
Microstream™ capnography monitoring
RespArray™ patient monitor
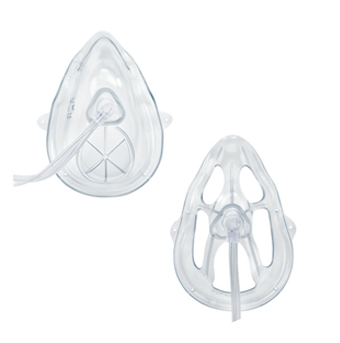
Oxy₂Pro™* mask with Microstream™ connector
Endoscopy
Nexpowder™* endoscopic hemostasis system
ManoScan™ ESO high resolution manometry system
Endoflip™ 300 impedance planimetry system
Bravo™ calibration-free reflux testing system
Transforming esophageal care
Talk with our team
Get in touch with a Medtronic product expert to find the right solutions for you, your patients, and your facility.
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic
Capnography monitoring system should not be used as the sole basis for diagnosis or therapy and is intended only as an adjunct in patient assessment.
TM* Third party brands are trademarks of their respective owners.
©2025 Medtronic. All rights reserved. Medtronic, Medtronic logo and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. US-DPG-2500173