The Society of Thoracic Surgeons 59th Annual Meeting: San Diego
San Diego Convention Center
January 21 – 23, 2023
Learn how we are transforming the field of lung health at STS, booth 1301.
Stop by our booth to:
- Experience our trusted portfolio and latest technologies in the lung cancer continuum of care
- Ask questions and engage with our team of product experts
- See a patient management platform demo
- Experience hands-on demo for our navigation bronchoscopy platform
- Ask questions and engage with our team of product experts
Join our live events at STS 2023
Defining the Role of Sub-lobar Resections in the Modern Era
Join us for a panel discussion moderated by Dr. Anthony Kim featuring Dr. David Cooke, Dr. Scott Atay, and Dr. Mark Onaitis as they consider the role of today’s sub-lobar resection from wedge to simple and complex segmentectomies, including lesions greater than 2 cm, and where segmentectomies fit in the modern era of neoadjuvant and target therapy.
Sunday, January 22, 7:00 p.m.
Featured at STS 2023, booth 1301: Medtronic Lung Health Solutions Product Portfolio
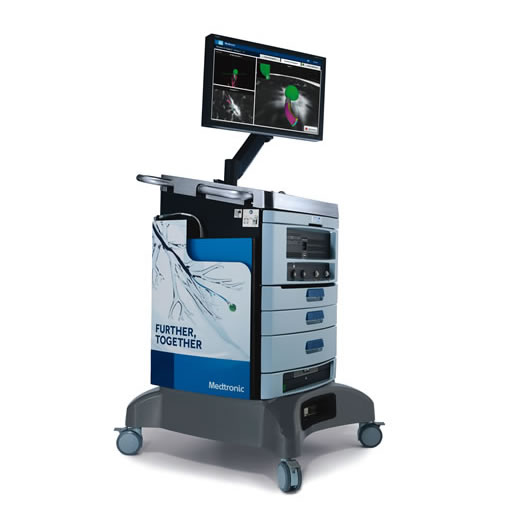
ILLUMISITE™ fluoroscopic navigation platform
Not only is the ILLUMISITE™ platform the leading electromagnetic lung navigation platform that corrects for CT-to-body divergence — it’s the only platform that uses digital tomosynthesis to do so.1,2 ILLUMISITE™ platform is clinically proven to help you accurately navigate lung nodules and designed for multidirectional sampling for a more thorough biopsy.1-4,†
LungGPS™ Patient Management Platform
Designed to assist clinicians in identifying incidental nodules and monitoring lung nodule patients, manage workflows, streamline thoracic tumor board collaboration, and provide analytics.
LigaSure ™ Maryland Jaw Thoracic Sealer/Divider
with Nano-coating
A vessel sealer designed for pulmonary vasculature.5-9,‡ The first minimally invasive LigaSure™ device designed for VATS procedures and indicated for sealing pulmonary veins and arteries up to and including 7 mm.5–10,‡,§
SigniaTM Small Diameter Reload
The Signia™ small diameter reload is a true 8 mm reload that can improve access and reach in your thoracic procedures.11,‡,Ω
Meet the team
Get in touch with a Medtronic product expert or your local account manager to find the right solutions for you, your patients, and your facility.
† Not evaluated in lesions <10 mm.
‡ May not be indicative of clinical performance.
§ As of December 20, 2022, based on indications for use for laparoscopic LigaSure™ devices.
Ω Compared to the Ethicon Echelon Flex™* powered vascular stapler.
The ILLUMISITE™ platform is indicated for displaying images of the tracheobronchial tree to aid the physician in guiding endoscopic tools or catheters in the pulmonary tract and to enable marker placement within soft lung tissue. It does not make a diagnosis and is not an endoscopic tool. Not for pediatric use.
WARNING: The ILLUMISITE™ platform may only be used by a qualified bronchoscopist.
CONTRAINDICATIONS: Flexible bronchoscopy should be performed only when the relative benefits outweigh the risks.
Absolute contraindications include, but are not limited to: lack of adequate facilities and personnel to care for emergencies such as cardiopulmonary arrest, pneumothorax, or bleeding, inability to adequately oxygenate the patient during the procedure.
Conditions that are usually considered absolute contraindications, unless risk-benefit assessment warrants the procedure: Coagulopathy or bleeding diathesis that cannot be corrected, severe obstructive airways disease, severe refractory hypoxemia, unstable hemodynamic status including dysrhythmias. Relative contraindications or conditions involving increased risk include but are not limited to: Recent myocardial infarction or unstable angina, uremia and pulmonary hypertension, and known or suspected pregnancy. Please refer to Instructions for Use for complete contraindication and risk information.
The LungGPS™ patient management platform is only available for sale in the United States.
The LungGPS™ patient management platform is an information management software only and does not diagnose or treat lung cancer nor replace lung cancer assessment in standard clinical practice. LungGPS™ should be used in conjunction with professional guidelines for patient management decision.
CONTRAINDICATIONS: LungGPS™ is intended to integrate with specific file output of sequencing instruments such as Illumina sequencers as well as integrate with specific electronic medical records and external database warehouses. The system should not be used with any instruments that have not been tested and verified as compatible with LungGPS™.
1. Pritchett MA, Bhadra K, Calcutt M, Folch E. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis 2020;12(4):1595–1611.
2. Medtronic financial and market analysis as of November 2021 of ENB and robotic lung navigation systems in market demonstrating Medtronic leading market share as defined by percentage of capital system sales.
3. Avasarala SK, Roller L, Katsis J, et al. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-Time Target Acquisition. Respiration. 2022;101(2):166-173. doi:10.1159/000518009
4. Dunn BK, Blaj M, Stahl J, Speicher J, Anciano C, Hudson S, Kragel EA, Bowling MR. Evaluation of electromagnetic navigational bronchoscopy using tomosynthesis-assisted visualization, intraprocedural positional correction and continuous guidance for evaluation of peripheral pulmonary nodules. J Bronchology Interv Pulmonol. 2022.
5. Based on internal report #RE00138840, LIG-45 memo, device length recommendation, thoracic (LF1930T). Feb. 6, 2018.
6. Based on internal test report #RE00125866, Jaw force and gap range burst pressure evaluation of EB4 thoracic Maryland device (LF1930T); conducted on bovine tissue. Nov. 20–21, 2017 and Nov. 27–30, 2017.
7. Based on internal test report #RE00134865, Burst pressure verification of pulmonary bovine veins (LF1930T). Jan. 17-18, 2018.
8. Based on internal test report #RE00122515, Verification of the LigaSure™ LF1930T device in a GLP chronic hemostasis canine study on pulmonary vasculature. Jan. 8–10, 2018.
9. Based on internal test report #RE00128442, GLP acute pulmonary vasculature hemostasis verification study of the LF1930T in hounds. Dec. 8, 2017.
10. Based on internal report #RE00147462, Pulmonary sealing claims for the LigaSure ™ LF1930T device (memo). March 29, 2018.
11. Based on report #RE00142825, Image creation for Signia™ small diameter reload. March 26, 2019.
©2023 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 12/2022–US-RE-2200691
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic