Unique screw design tacks for strong mesh fixation.1,2
The AbsorbaTack™ fixation device — complementing an unparalleled hernia repair portfolio
Providing the standard of hernia care your patients need depends on the fixation solutions you have at hand.
For more than 20 years, surgeons have trusted the AbsorbaTack™ fixation device for secure temporary mesh fixation,1,2 patient comfort, and clinician peace of mind.4
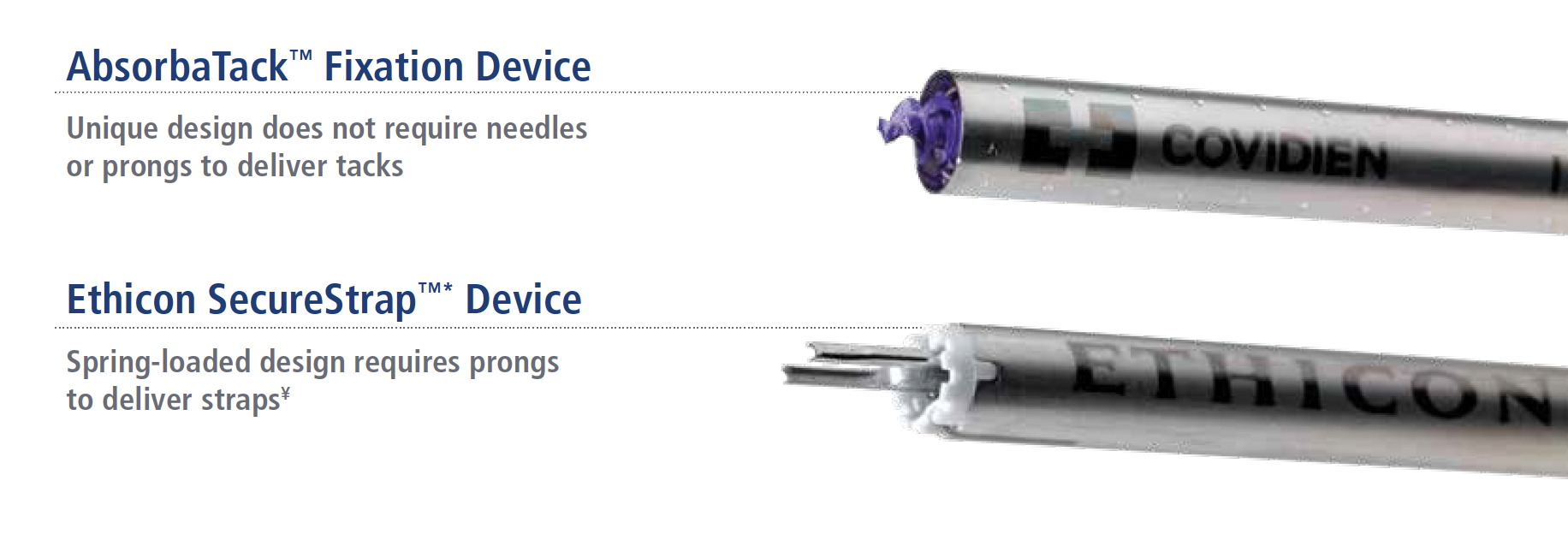
Designed with absorbable self-tapping technology, our fixation device securely holds the mesh in place1 while your patient heals.3 With a unique screw design offering needleless tack delivery, our fixation device makes needle stick injuries a thing of the past.

Patient comfort,1–3,5 strong fixation,1,3,5 peace of mind.
The AbsorbaTack™ family of products deliver mesh fixation essentials physicians can see. You can trust you're deploying tacks successfully with a reliable tack deployment and a ratcheted mechanism delivering tactile feedback while you fire.
Innovative Design
- Put control in your hands with the unique screw-like design of the AbsorbaTack™ fixation device.
- The AbsorbaTack™ device doesn’t need a sharp piloting needle to deploy, eliminating the risk of inadvertent needlesticks in the OR.
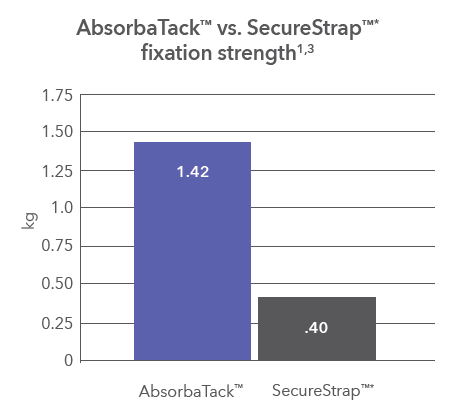
Fixed on fixation strength
Strong mesh fixation1,2 is at the forefront of the AbsorbaTack™ fixation device design.
Our absorbable fixation device fastens mesh to soft tissue during surgery and remains for weeks while healing is underway.4,† Its smooth head with 5.1-mm proximal wings provides the surface area to sit flush against the mesh and secure it firmly in place.1
Putting patient comfort first
Keeping the mesh securely in place may decrease mesh4 shift during patient healing, which is vital to hernia recovery.
But the benefits of mesh fixation with our device go beyond that. By design, the device supports a minimally invasive approach through a 5-mm shaft.
And the absorbable tack ensures no foreign materials remain in the body over time 5,‡ because there is no metal required for mesh fixation. The result — tacks that vanish and mesh that stays in place.

Delivering peace of mind
With the AbsorbaTack™ fixation device, you’ll discover many benefits for effective hernia repair using absorbable fixation to secure your mesh.
Control meets confidence with a unique screw design that promotes controlled deployment into tissue by allowing the tack to self-tap. With no need for piloting needles to place the tack, there’s no risk for needle sticks.
The absorbable tacks supply real staying power throughout the healing process with potentially fewer adhesions than permanent tack fixation.4,†

Here to help
Ready to see the difference the AbsorbaTack™ fixation device can make in your healthcare setting?
Our Medtronic representatives have the knowledge and resources to help you make it part of your hernia
repair routine.
†Animal data may not correlate with human clinical outcomes.
‡ Bench test results may not be indicative of clinical performance.
1. Colak E, Ozlem N, Kucuk O. Prospective randomized trial of mesh fixation with absorbable versus nonabsorbable tacker in laparoscopic ventral incisional hernia repair. Int J Clin Exp Med. 2015;8(11):21611-21616.
2. Based on internal report #TD1025, Mechanical evaluation of AbsorbaTack™, ProTack™, and SECURESTRAP™* in shear mode using foam media. January 2012.
3. Based on internal test report #R2165-055-1, mesh overlap claims testing report. Test performed in a simulated bench model. Results may not correlate to performance in animal or cadaveric tissue, or performance in humans. P-value= 0.007 March 2014.
4. Hollinsky, C., Kolbe, T., Walter, I. et al. Tensile strength and adhesion formation of mesh fixation systems used in laparoscopic incisional hernia repair. Surg Endosc. 2010 Jun;24(6):1318-1324.
5. Based on internal report # RE00003170-2, ReliaTack™ Phase II In-vitro Mass Loss. July 2016.
© 2022 Medtronic. All rights reserved. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic.™* Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. 01/2022 – US-HR-2100155 (WF#5947988)
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2026 Medtronic