34th Annual ELSO Conference
September 28th - October 1st, 2023
Hyatt Regency Seattle
Seattle, WA
Stop by booth #307 to see how we’re advancing ECLS every day!
Medtronic Hands-on ECLS Workshop
At ELSO, Medtronic will be offering two hands-on workshops with our pediatric and adult cannulae and ECLS therapy options. Come learn and engage with fellow clinicians in hands-on ECLS simulation and product workshop.
When:
Friday, September 29 from 9-11 a.m.
Saturday, September 30 from 1-3 p.m.
Where:
Hyatt Regency
Room #305
Pediatric Cannulation Simulation with Dr. Brian Gray and Dr. Sarah Keene
Featuring Crescent™* RA Jugular Dual Lumen Catheter

Brian W. Gray, M.D.
Assistant Professor of Pediatric Surgery
Surgical Director of ECMO
Program Director, Pediatric Surgery Fellowship
Riley Hospital for Children
Indiana University School of Medicine

Sarah D. Keene M.D.
Associate Professor of Pediatric Medicine
Medical Director – Neonatal ECMO
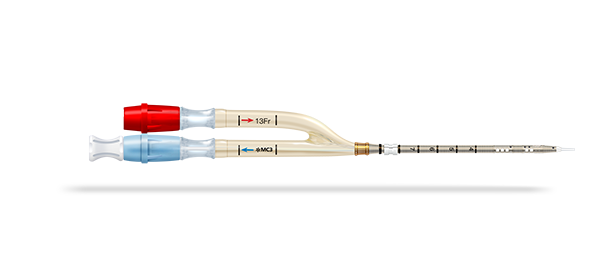
The Crescent™* RA jugular dual lumen catheter offers a right atrial design and includes both an introducer for a percutaneous technique and a blunt-tip obturator for a surgical cut-down technique
Adult Cannulation Simulation with Dr. John Gurley
Featuring Crescent™* Jugular Dual Lumen Catheter and
Bio-Medicus Life Support™ Catheters and Introducers

John Gurley M.D.
Professor of Medicine, Cardiology
Director of Structural Heart Program
University of Kentucky HealthCare
Gill Heart Institute

The Crescent™* catheter is the first FDA-cleared, jugular dual lumen long-term ECMO catheter. It allows for more accurate placement with just one cannulation site, delivers enhanced flow dynamics, and helps maintain optimal flow1 once placed.

Developed with extensive user input, the Bio-Medicus Life Support™ catheter offers more lengths, sizes, and configurations than any other brand — giving you the choices you need to individualize your patients' ECLS care.
ECMO Simulation with Dr. Colin McCloskey
Featuring Nautilus™* Smart ECMO Module

Colin McCloskey M.D.
Associate Professor of Emergency Medicine and Anesthesia Critical Care
Case Western Reserve University School of Medicine
University Hospitals Cleveland Medical Center ECMO Services
Simplify your circuit with the first ECMO oxygenator featuring integrated monitoring. The Nautilus™* Smart ECMO Module improves long-term gas transfer1 while providing real-time device performance data accessible from an intuitive touch screen.

Risks of ECLS include heart, vessel, or lung damage, hypoxia, anemia, infection, hemorrhage, liver or kidney failure, stroke, and death.
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic
Nautilus™* Smart ECMO Module is manufactured by MC3, Inc., and exclusively distributed by Medtronic.
Crescent™* jugular dual lumen is manufactured by MC3, Inc. and exclusively distributed by Medtronic.
Crescent™* RA jugular dual lumen catheter is manufactured by MC3, Inc. and exclusively distributed by Medtronic.
1 Design verification and validation data on file at MC3. These tests may not be indicative of clinical performance.
Nautilus™* Smart ECMO Module Important Safety Information
Only clinicians thoroughly trained in extracorporeal life support procedures should use this device. Read all warnings, precautions, and Instructions for Use carefully prior to use. Failure to read and follow all instructions, or failure to observe all stated warnings, could cause serious injury or death to the patient.
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Crescent™* and Crescent™* RA Jugular Dual Lumen Catheters Important Safety Information
Care and caution should be taken to avoid damage to vessels and cardiac tissue during cannulation or other cardiac surgery procedures. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use.
Only physicians with previous training and experience with venous catheterization and extracorporeal life support should use this device.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Crescent™* and Crescent™* RA jugular dual lumen catheters are not approved in every geography.
Bio-Medicus Life Support™ Important Safety Information
- Only physicians trained and experienced in using percutaneous catheterization techniques (such as the Seldinger technique), ECMO, and ECLS should use this device.
- Note: The benefits of catheterization for extracorporeal circulation must be weighed against the risk of systemic anticoagulation and subsequent propensity for hemorrhage.
- Caution: Ensure that the catheter size selected is of adequate size for the vessel to allow distal perfusion of the limb when the catheter is in place. Improper catheter size may be difficult to advance. The vessel must be large enough to ensure perfusion and venous return.
Care and caution should be taken to avoid damage to vessels and cardiac tissue during cannulation or other cardiac surgery procedures. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
© 2023 Medtronic. All rights reserved. Medtronic, Medtronic logo and engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. ™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company.


