ATS 2024 International Annual Conference
San Diego - May 19-21, 2024
Countdown to a new era in lung biopsy
Has bronchoscopy finally replaced CT-guided biopsy? Join the “New Frontiers in Lung Cancer: Toys, Tools and Treatments” mini symposium on Tuesday, May 21, 2024 at 2:15 PM – 4:15 PM to hear the preliminary results of the ground-breaking VERITAS randomized controlled trial.
Learn how we are transforming lung health at booth 3007
Stop by our booth to:
- Experience the system behind VERITAS
- Test your skills with our ENB virtual reality experience
- Ask us about VERITAS!
Join our live events at ATS 2024
Innovation Hub #3: VERITAS RCT: Has bronchoscopy finally replaced CT-guided biopsy?
Despite its high diagnostic yield, transthoracic needle aspiration (TTNA) has been associated with higher complication rates compared to electromagnetic navigational bronchoscopy (ENB).1,2 Join our discussion as we delve into the implications of the latest clinical data, exploring how the power of fluoroscopic navigation can help you build a sustainable lung biopsy program.
Monday May 20th 12:15 p.m. to 12:35 p.m.
New Frontiers in Lung Cancer: Toys, Tools and Treatments
Hear from Dr. Robert Lentz, as he discusses the abstract: Navigational Bronchoscopy Versus Computed Tomography-guided Transthoracic Needle Biopsy for the Diagnosis of Indeterminate Lung Nodules: Initial Results from the VERITAS Multicenter Randomized Trial.
Tuesday May 21st 2:15 p.m. to 4:15 p.m.
Featured at ATS 2024, booth 3007:
Medtronic Lung Health Solutions innovative product portfolio

Seeing is Believing — Experience a virtual reality ILLUMISITE™ fluoroscopic navigation platform procedure to see the difference!
Join us in the booth to experience the ILLUMISITE™ platform with virtual reality!
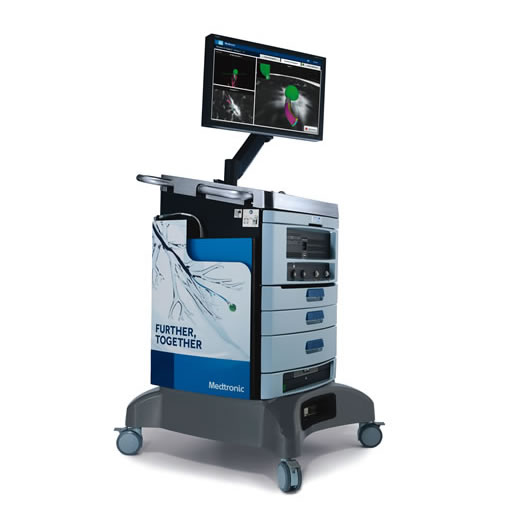
ILLUMISITE™ Platform
Clinically proven to help you accurately navigate to lung nodules with its unique fluoroscopic navigation technology utilizing digital tomosynthesis, the ILLUMISITE™ platform is designed to provide the diagnostic yield you rely on.3,4,5,†,‡
† Based on evidence from a single-center prospective study including a total of 82 consecutive patients
‡ Based on evidence from a single-center retrospective study including a total of 72 consecutive patients
Biopsy Tools
The ILLUMISITE™ platform is designed to sample multi-directionally for a more thorough biopsy. Take your pick from our expansive biopsy tool portfolio, whether you want to use the Arcpoint™ pulmonary needle with its ability to minimize deflection of the extended working channel6 or the GenCut™ core biopsy system with its unique blade design intended to cut core tissue samples from lung lesions.7
Meet the team
Get in touch with a Medtronic product expert or your local account manager to find the right solutions for you, your patients, and your facility.
1. DiBardino, D. M., Yarmus, L. B., & Semaan, R. W. (2015). Transthoracic needle biopsy of the lung. Journal of thoracic disease, 7(Suppl 4), S304–S316.
2. Folch, E. E., Labarca, G., Ospina-Delgado, D., Kheir, F., Majid, A., Khandhar, S. J., & Fernandez-Bussy, S. (2020). Sensitivity and safety of electromagnetic navigation bronchoscopy for lung cancer diagnosis: systematic review and meta-analysis. Chest, 158(4), 1753-1769.
3. Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic Navigation Bronchoscopy With Tomosynthesis-based Visualization and Positional Correction: Three-dimensional Accuracy as Confirmed by Cone-Beam Computed Tomography. J BronchologyIntervPulmonol. 2021;28(1):10-20. doi:10.1097/LBR.0000000000000687
4. Avasarala SK, Roller L, KatsisJ, et al. Sight Unseen: Diagnostic Yield and Safety Outcomes of a Novel Multimodality Navigation Bronchoscopy Platform with Real-time Target Acquisition. Respiration. Published online September 03, 2021. doi: 10.1159/000518009.
5. Dunn BK, Blaj M, Stahl J, Speicher J, Anciano C, Hudson S, Kragel EA, Bowling MR. Evaluation of electromagnetic navigational bronchoscopy using tomosynthesis-assisted visualization, intraprocedural positional correction and continuous guidance for evaluation of peripheral pulmonary nodules. J Bronchology Interv Pulmonol. 2022.
6. Based on internal report #DRE00331, EDGE EWC catheter tip deflection study for GenCut™ core biopsy system, CrossCountry™ transbronchial access tool, and Arcpoint™ pulmonary needle. January 2017.
7. Based on internal test report #ADO110, A chronic, GLP study to evaluate the usability and safety of the coring tool in a porcine model. September 2014. Data on file
The ILLUMISITE™ platform is indicated for displaying images of the tracheobronchial tree to aid the physician in guiding endoscopic tools or catheters in the pulmonary tract and to enable marker placement within soft lung tissue. It does not make a diagnosis and is not an endoscopic tool. Not for pediatric use. WARNING: The ILLUMISITE™ platform may only be used by a qualified bronchoscopist. CONTRAINDICATIONS: Flexible bronchoscopy should be performed only when the relative benefits outweigh the risks. Absolute contraindications include, but are not limited to: lack of adequate facilities and personnel to care for emergencies such as cardiopulmonary arrest, pneumothorax, or bleeding, inability to adequately oxygenate the patient during the procedure. Conditions that are usually considered absolute contraindications, unless risk-benefit assessment warrants the procedure: Coagulopathy or bleeding diathesis that cannot be corrected, severe obstructive airways disease, severe refractory hypoxemia, unstable hemodynamic status including dysrhythmias. Relative contraindications or conditions involving increased risk include but are not limited to: Recent myocardial infarction or unstable angina, uremia and pulmonary hypertension, and known or suspected pregnancy. Please refer to Instructions for Use for complete contraindication and risk information.
©2024 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 04/2024 US-RE-2400178
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic