Medtronic Cardiac Surgery at CREF 2023
42nd Cardiothoracic Surgery Symposium
September 13-16, 2023
Marriott Marquis San Diego Marina
San Diego, CA USA
Visit booth #15 in the exhibit hall to see how we’re putting patients first by elevating ECLS therapy and Perfusion care every day.
Stop by our booth to:
- Learn more about our latest technology advances
- See our full portfolio of ECMO, Perfusion and Autotransfusion products
Wednesday, September 13
Miramar | Third Floor, South Tower
8:30 AM – 9:30 AM Pacific,
9:30 AM – 10:30 AM Pacific,
11:00 AM – 12:00 PM Pacific
Hands-on Workshop
Experience Transformative Innovation in ECMO, Cannula,
and Autotransfusion
Connect with Medtronic during a hands-on ECLS workshop featuring Nautilus™* Smart ECMO Module, Crescent™* and Crescent™* RA jugular dual lumen catheters, and Bio-Medicus Life Support™ catheters. Additionally, we’ll have a hands-on learning experience with the autoLog IQ™ Autotransfusion System.
Friday, September 15
Marina Ballroom DE | Third Floor, South Tower
12:30 PM – 2:00 PM Pacific
Product Theater
Changing the Shape in ECMO Oxygenation
Come enjoy a plated lunch and listen to your fellow health care providers from two large ECMO centers as they highlight the integration and use of the Nautilus™* ECMO Oxygenator and Nautilus™* Smart ECMO Module in their programs.
Faculty:
Martha McBride, MSN, CRNP
Director of ECMO, Children’s of Alabama
Natasha Crain, BSN, RN, CCRN, CES-A
ECMO Operations Manager, UK HealthCare
ECLS Solutions
Nautilus Smart ECMO Module
Simplify your circuit with the first ECMO oxygenator featuring integrated monitoring. The Nautilus™* Smart ECMO Module improves long-term gas transfer1 while providing real-time device performance data accessible from an intuitive touch screen.
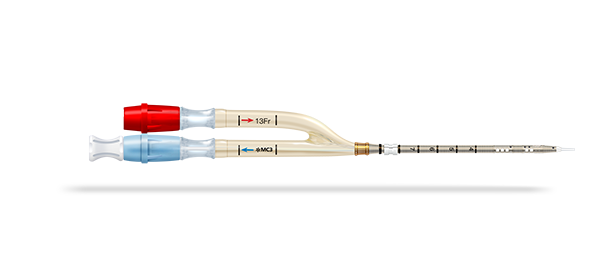
Crescent Jugular Dual Lumen Catheter
The Crescent™* catheter is the first FDA-cleared, jugular dual lumen long-term ECMO catheter. It allows for more accurate placement with just one cannulation site, delivers enhanced flow dynamics, and helps maintain optimal flow1 once placed.
Crescent RA Jugular Dual Lumen Catheter
We understand the value of VV ECMO therapy, and now you have the option to provide your pediatric patients with the same game-changing flow1 you expect from the Crescent™* catheter name. The Crescent™* RA jugular dual lumen catheter offers a right atrial design and includes both an introducer for a percutaneous technique and a blunt-tip obturator for a surgical cut-down technique.
Bio-Medicus Life Support Catheter and Introducers
Developed with extensive user input, the Bio-Medicus Life Support™ catheter offers more lengths, sizes, and configurations than any other brand — giving you the choices you need to individualize your patients' ECLS care.
Risks of ECLS include heart, vessel, or lung damage, hypoxia, anemia, infection, hemorrhage, liver or kidney failure, stroke, and death.
Blood Management Innovations
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic
Nautilus™* ECMO Oxygenator and Nautilus™* Smart ECMO Module are manufactured by MC3, Inc., and exclusively distributed by Medtronic.
Crescent™* jugular dual lumen is manufactured by MC3, Inc. and exclusively distributed by Medtronic.
Crescent™* RA jugular dual lumen catheter is manufactured by MC3, Inc. and exclusively distributed by Medtronic.
1 Design verification and validation data on file at MC3. These tests may not be indicative of clinical performance.
Nautilus™* Smart ECMO Module Important Safety Information
Indications: The Nautilus™* Smart ECMO Module with integrated heat exchanger is intended to provide assisted long-term extracorporeal circulation and physiologic gas exchange (oxygenation and carbon dioxide removal) of the patient’s blood for up to 48 hours in adult and pediatric adolescent patients with acute respiratory failure or acute cardiopulmonary failure where other available treatment options have failed and continued clinical deterioration is expected or the risk of death is imminent. The integrated heat exchanger is intended to heat or cool the blood as needed during use. Integrated fluid path pressure, temperature, and oxygen saturation monitoring are achieved by built-in sensor modules and display.
Warnings: Only clinicians thoroughly trained in extracorporeal life support procedures should use this device. Read all warnings, precautions, and Instructions for Use carefully prior to use. Failure to read and follow all instructions, or failure to observe all stated warnings, could cause serious injury or death to the patient.
For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Crescent™* and Crescent™* RA Jugular Dual Lumen Catheters Important Safety Information
Indications For Use: The Jugular Dual Lumen Catheter is a single use dual lumen catheter, which provides both venous drainage and reinfusion of blood via the jugular vein, that is indicated for use in adult and pediatric patients with acute respiratory failure requiring Veno-Venous Extracorporeal Membrane Oxygenation where other available treatment options have failed and continued clinical deterioration is expected or the risk of death is imminent.
Contraindication: This device is not designed, sold, or intended for use except as indicated.
Warnings and Precautions
Warning: Do not use without adequate systemic anticoagulation.
Warning: Use of the Jugular Dual Lumen Catheter beyond the duration established by the available in vitro and in vivo testing has not been demonstrated. Please refer to the Instructions for Use (sect. 9 & 14) for a summary of bench and animal studies performed and for the possible clinical observations that may necessitate or predict the need for device replacement/change-out throughout the duration of use for this device.
Care and caution should be taken to avoid damage to vessels and cardiac tissue during cannulation or other cardiac surgery procedures. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use.
Only physicians with previous training and experience with venous catheterization and extracorporeal life support should use this device.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
Crescent™* and Crescent™* RA jugular dual lumen catheters are not approved in every geography.
Bio-Medicus Life Support™ Important Safety Information
- Only physicians trained and experienced in using percutaneous catheterization techniques (such as the Seldinger technique), ECMO, and ECLS should use this device.
- Note: The benefits of catheterization for extracorporeal circulation must be weighed against the risk of systemic anticoagulation and subsequent propensity for hemorrhage.
- Caution: Ensure that the catheter size selected is of adequate size for the vessel to allow distal perfusion of the limb when the catheter is in place. Improper catheter size may be difficult to advance. The vessel must be large enough to ensure perfusion and venous return.
Care and caution should be taken to avoid damage to vessels and cardiac tissue during cannulation or other cardiac surgery procedures. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician.
autoLog™ Autotransfusion System Important Safety Information
Intended use: The autoLog IQ autotransfusion system and Medtronic wash kit are intended for use in the collection, concentration, washing, and reinfusion of autologous blood. Areas of application may include, but are not limited to, the following:
• Surgeries including general, cardiovascular, orthopedic, vascular, plastic/reconstructive, obstetric/gynecologic, and neurological
• Postoperative treatment areas
Contraindications: The process of blood recovery is associated with few complications. The surgical team must consider the risks and relative contraindications of autotransfusion in any surgical procedure before proceeding with autotransfusion.
Warnings: Do not use autoLog for direct patient reinfusion. Adequate safeguards do not exist to protect patients in these situations. Do not directly reinfuse processed blood from the holding bag to the patient. Directly reinfusing the blood from the holding bag exposes the patient to the risk of possible air embolism. Never transfuse blood that is suspected of having high hemolysis.
Caution: Federal law (USA) restricts this device to sale by or on the order of a physician. For a complete listing of indications, contraindications, precautions and warnings, please refer to the Instructions For Use which accompany each product.
© 2023 Medtronic. All rights reserved. Medtronic, Medtronic logo and engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. ™*Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. MAJ_75020