

NEW
Dextile™ anatomic mesh
The right balance of anatomic shape, increased coverage size†,
porosity and strength.1-5
Our goal is to give real-world surgeons a real-world solution that improves ease of handling while improving anatomical compliance.
Learn MoreDextile™ anatomical mesh is a product of a collaboration with more than 100 HCPs worldwide. Clinician feedback helped us create this unique, truly anatomical mesh to adapt with the contours of the inguinal anatomy.1
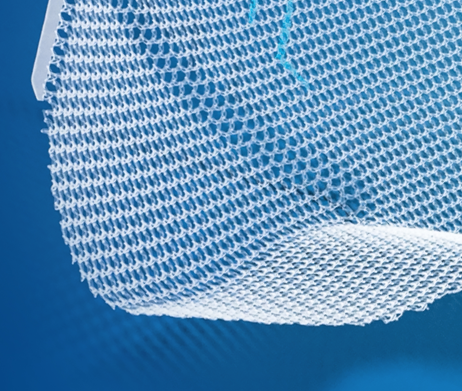
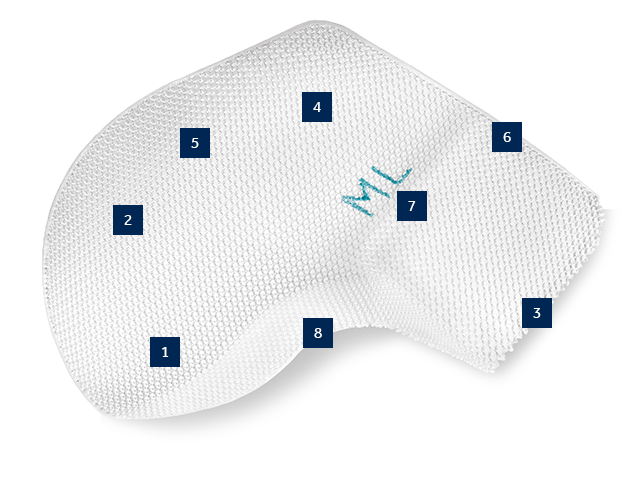
A truly anatomical polypropylene mesh with a patented 3-D shape that conforms to contours of the inguinal region.1,6 Dextile™ anatomical mesh provides wide coverage of the myopectineal orifice, minimizing the risk of recurrence.3,4,7And with large pores for excellent tissue integration and improved visualization5,8,9,‡ - it’s just right.
Designed specifically for minimally invasive inguinal hernia repair, including conventional TAPP and TEP and TAPP robotic techniques.11 Dextile™ mesh has the right combination of qualities for equal or better intraoperative handling compared to the competition.
† Compared to Bard 3DMax™* and Bard 3DMax™* Light.
‡ Animal data may not correlate with human clinical outcomes.
Ω Based on cadaver lab testing involving twelve U.S. and EU surgeons with strong 3DMAX™* and 3DMAX™* light experience.
§ Ease of use includes trocar introduction, deployment and positioning.
REFERENCES:
1. Based on internal test report #43008CR268. Design output file Merlin. February 2019.
2. Based on internal test report#43008CR315, Dextile™ anatomical mesh vs 3DMax™* Light mesh: mesh physical and mechanical comparison. August 2019.
3. Based on internal report #43008CR339, Support for marketing claims related to surface of Dextile™ anatomical mesh vs. competitors. August 2019.
4. Based on internal report #Dextile™ anatomical mesh, Surgeon interview report. July 2019.
5. Based on internal report #43008CR336, Support for marketing claims related to the Dextile™ anatomical mesh transparency. August 2019.
6. Based on Medtronic patent #US-20180318057-A1, Prosthesis for inguinal hernia repair. November 2018.
7. HerniaSurge Group. International guidelines for groin hernia management. Hernia. 2018 Feb;22(1):1-165.
8. Weyhe D, Cobb W, Lecuivre J, et al. Large pore size and controlled mesh elongation are relevant predictors for mesh integration quality and low shrinkage – Systematic analysis of key parameters of meshes in a novel minipig hernia model. Int J Surg. 2015;22: 46–53. July 2015.
9. Based on Phycher report #IMP-Toxsys-1 month-PH-19-0218 final report. August 2019.
10. Based on internal report #43008CR316a, Evaluation of Dextile™ anatomical mesh through human cadaver lab-PRL design validation. July 2019.
11. Based on internal report #43008CR343, Robotically assisted inguinal hernia surgery–review considering Dextile™ anatomical mesh. September 2019.
12. Based on internal report #43008CR326, Support for marketing claims related to Dextile™ anatomical mesh handling. August 2019.
13. Based on internal report #43008CR344, Support to marketing claim related to Dextile™ anatomical mesh ease of use vs Bard 3DMax™* light. August 2019.