
Conecte-se ao nosso ecossistema cirúrgico
que evolui de acordo com suas necessidades.
Oferecemos uma colaboração única em cirurgias abertas, laparoscópicas, minimamente invasivas (MIS) e assistidas por robótica, garantindo opções flexíveis para adaptar soluções à sua prática cirúrgica.
Com foco na colaboração, apoio, treinamento e inovação, nosso ecossistema cirúrgico garante que atendamos às exigências atuais e inovemos para as cirurgias minimamente invasivas do futuro.
Você sabe quais são os pilares no desenvolvimento do nosso portfolio?
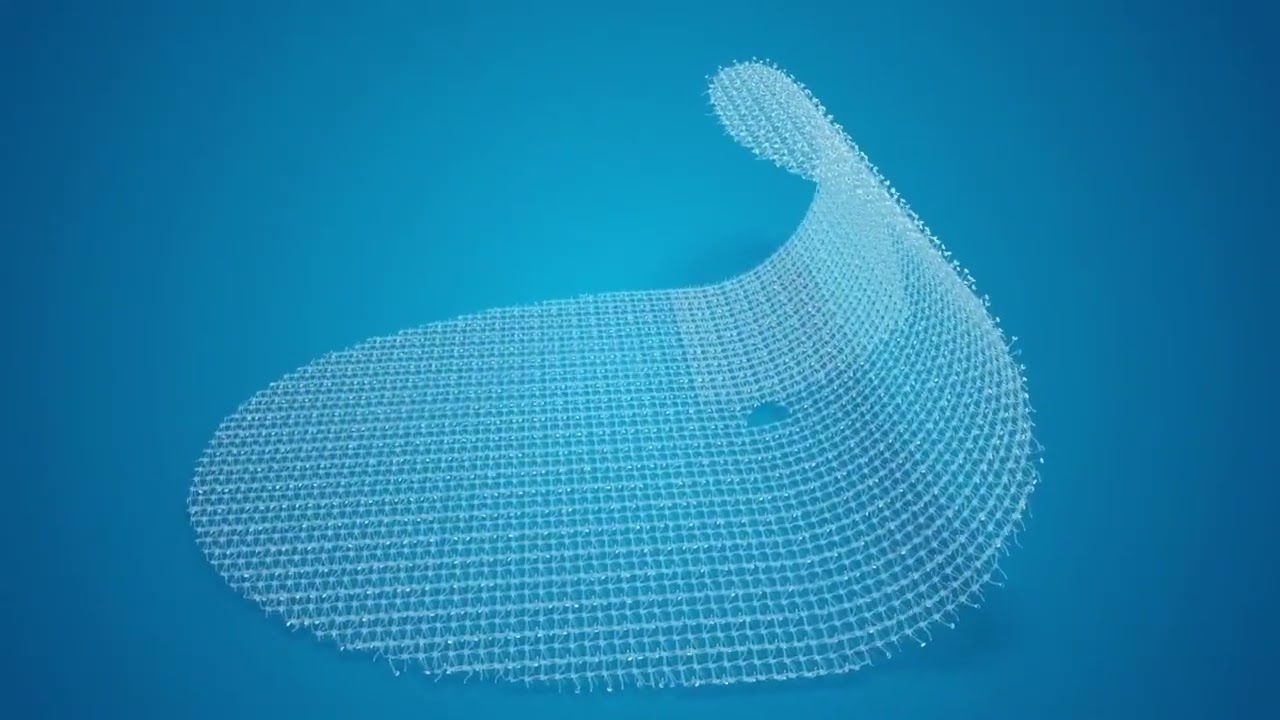
Descubra mais do portfólio para cirurgia de reparo de Hérnia da Medtronic
Portfólio para abordagem da hérnia inguinal aberta

ProGrip™ Aberta
Tela autofixante ProGrip™, uma tecnologia comprovada no reforço de tecidos por quase uma década.

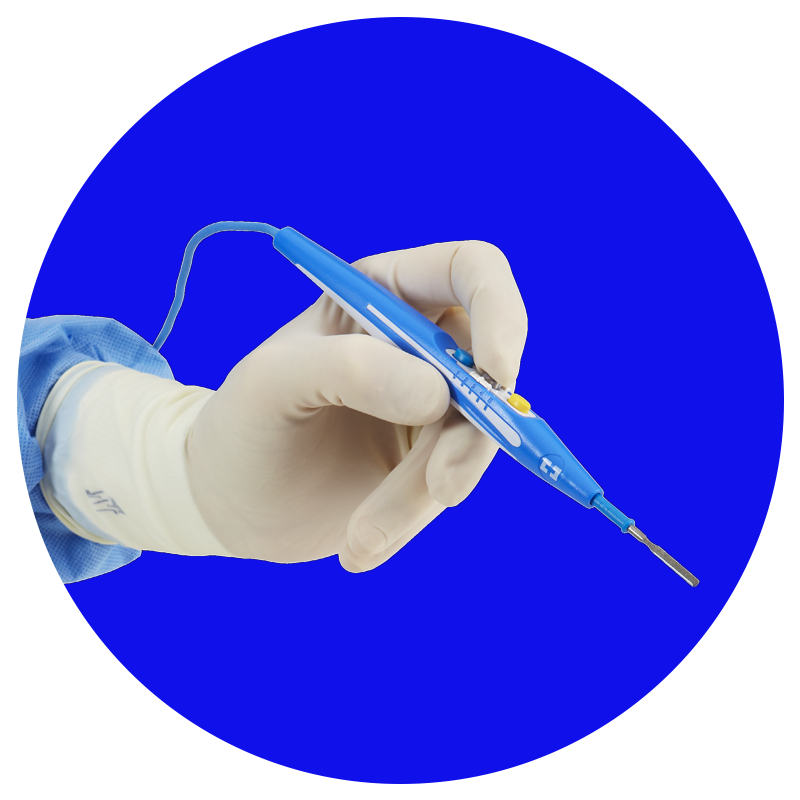
Force TriVerse™
Oferece menor dispersão térmica com uma combinação única de hemostasia monopolar e dissecção.
Portfolio de suturas
Medtronic tem um portfólio completo de soluções de suturas para o reparo de hérnia.
Portfólio para abordagem laparoscópica da hérnia inguinal
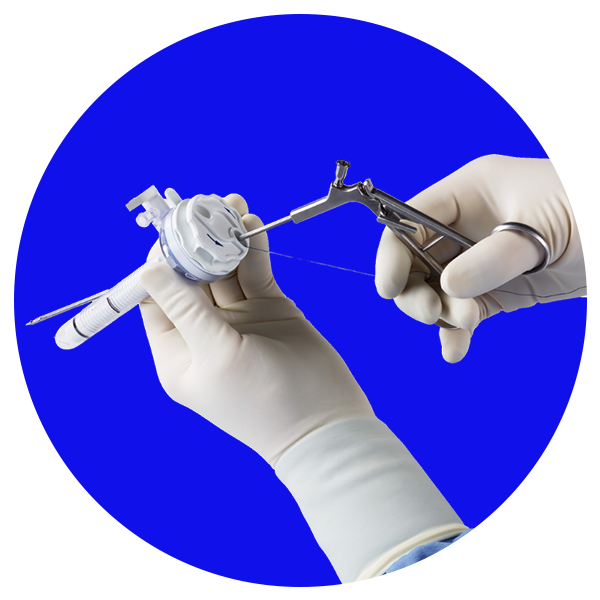
VersaOne™
Acesso, sem excessos. Uma plataforma de trocarte universal que oferece desempenho, opções e tranquilidade em uma solução completa.1,2

Progrip™ Laparoscópica
A tela laparoscópica Progrip™, com mais de 5.000 microganchos absorvíveis elimina a necessidade de fixação tradicional.3-8

Dextile™
A tela anatômica com estrutura 3D que mantém sua forma após a passagem pelo trocarte, facilitando o posicionamento e colocação adequados para se adaptar aos contornos da anatomia inguinal.
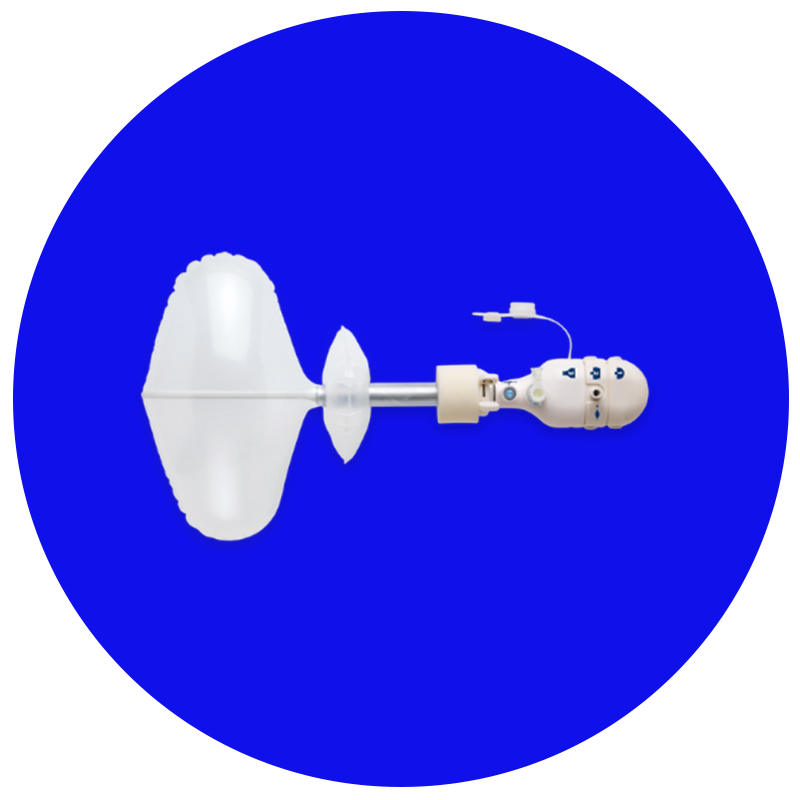
Spacemaker™
Sistema integrado que otimiza o acesso, dissecção e eficiência na abordagem TEP para correção de hérnia inguinal.9-12
Portafolio para abordagem de correção de hernia ventral IPOM.

Symbotex™
Tela composta de poliéster monofilamento para reforço do tecido da parede abdominal.14-22

Parietene™ DS
Tela composta de polipropileno para fortalecimento dos tecidos moles da parede abdominal, transparente.23-30
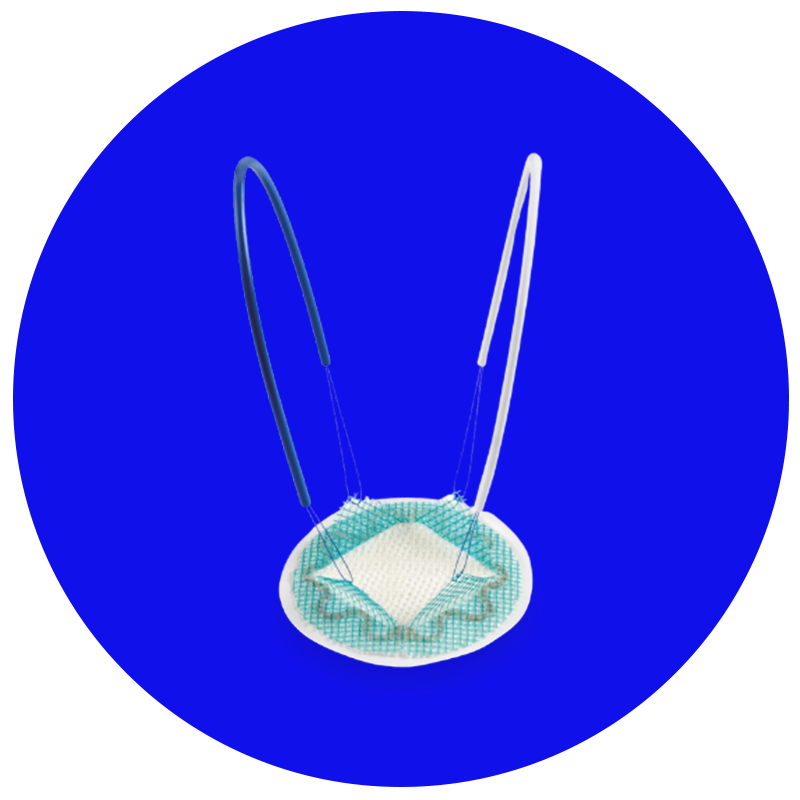
Parietex™ Composite Ventral Patch
Tela para reparo de pequenos defeitos ventrais, evita aderências com ótima integração.24-28

Maxon™ Maxsorb™
Suturas de longa absorção para uso na técnica de "small-bites" para evitar deiscências ao fechar a parede abdominal.

V-Loc™
Nosso dispositivo de fechamento de feridas sem necessidade de amarrar nós forte, seguro e rápido29
Portafolio para abordagem de hérnias ventrais complexas e pacientes obesos.

Force TriVerse™
Oferece menos dispersão térmica com uma combinação única de hemostasia monopolar e dissecção.40-43
Procedimentos mais complexos necessitam soluções avançadas para dissecção.

Sonicision™
Sistema sem fio de coagulação e disecção ultrassônica de vasos de até 5mm.42-43
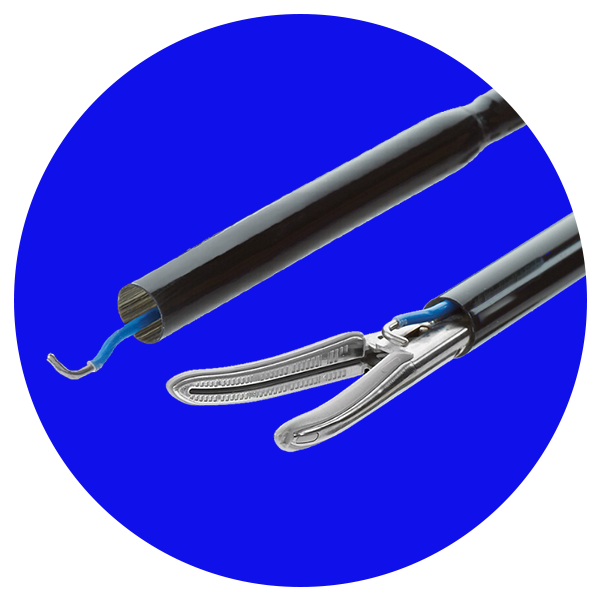
LigaSure™
Tecnologia eficiente que combina pressão e energia para criar fusão de vasos de até 7 mm.
Reduz a perda de sangue, o tempo de operação e a permanência do paciente.44-55

Signia™
Grampeador inteligente junto a sua escolha entre cargas inteligentes

V-Loc™
Nosso dispositivo de fechamento de feridas sem necessidade de amarrar nós forte, seguro e rápido.29
Referencias
1. Based on internal test report #RE00365735. Periscope II in vivo penetration/fixation competitive EBM test. In October 2021. 2. Based on VOC; Eichler Project Market Research Lieberman Research. March 22, 2013. 3. 16-emea-lap-progrip-technique-brochure-781498, Based on internal test report #0902CR123. June 2012. 4. Instructions for use of the ProGrip™ self-fixing laparoscopic mesh 5. Kolbe T, Hollinsky C, Walter I, Joachim A, Rülicke T. Influence of a new self-gripping hernia mesh on male fertility in a rat model. Surg Endosc. 2010;24(2):455-461. 6. Based on internal test report #TEX033a. October 2012. 7. Based on internal test report #98640. February 2012. 8. Based on internal test report #0902CR122. June 2012. 9. Based on internal project verification report #RE00010041, 10. Spacemaker™ Pro access and dissection system. December 2014. 11. Based on internal validation report #RE00013395, Spacemaker™ Pro access and dissection system. June 2015. 12. Spacemaker™ Pro [instructions for use]. Mansfield, Massachusetts: Medtronic;2014. 13. 11/2021- US-WC-2100106-[WF#5406475] 14. Demonstrated in a preclinical study sponsored by Covidien, carried out on pigs in May 2013 with 6 surgeons and aiming at validating the design of Symbotex™ composite mesh - Covidien internal report 0901CR252a (June 2013).15. Based on the results of the Covidien-sponsored preclinical study carried out on a porcine model to validate the design of Symbotex™ composite mesh - Covidien design validation report 0901CR249a (June 2013). 16. Definition of the Symbotex™ clinging effect observed during the design validation conducted by Covidien in a porcine model in May 2013 - Covidien internal memorandum 0901CR261a (July 2013). 17. Assessed in a preclinical study sponsored by Covidien, initiated in May 2013, using a porcine model to evaluate local tissue effects and tissue integration of Symbotex™ Composite mesh vs Parietex™ Optimized composite mesh after laparoscopic ventral repair - Namsa report No.163005 (October 2013). 18. Assessed in a preclinical study sponsored by Covidien, initiated in April 2013, using a rat caecal abrasion model and evaluating local tissue effects, tissue integration and minimizing tissue attachment performance of Symbotex™ composite mesh vs. Parietex™ Optimized composite mesh - Namsa report No.162750 (May 2013). 19. Evaluated in a preclinical study sponsored by Covidien, conducted in April 2013, and comparing local tissue effects and integration, collagen film degradation and tissue attachment performance of Symbotex™ composite mesh with Ventralight™* ST mesh and Physiomesh™* flexible composite mesh in a porcine model - Namsa report No.163905 (October 2013). 20. Comparison of the physical and mechanical properties of Symbotex™ composite mesh to those of Parietex™ optimized composite mesh through a bench study conducted by Covidien in July 2013 - Covidien internal report TEX043 (July 2013). 21. D. Weyhe, W. Cobb, D. Lomanto et al, Comparative analysis of the performance of a series of meshes based on weight and pore size in a novel mini-pig hernia model - EHS 2013 (SC130037). 22. Documented in the design verification report issued by Covidien in July 2013 - Covidien design verification report 0901CR247b (July 2013) 23. Based on internal surgeon labs for design validation #T2294CR208. Marketing VOC. Evaluation performed through users test and questionnaire in a simulated use environment using a porcine model (n=7). September 2016. 24. Based on internal preclinical report #T2294CR212, Design validation of Parietene™ DS composite mesh in surgeon labs: Feedback collected from seven independent surgeons in a simulated use environment using a porcine model. September 2016. 25. Based on internal test report #RAT207, Robotically assisted laparascopic ventral hernia repiar and Parietene™ DS composite mesh. November 2018. 26. Based on NAMSA study #197165. Thirteen week systemic toxicity and local tissue effects study in rats following subcutaneous implantation. August 2016. 27. Based on internal test report #T2294CR225, Comparison of nonabsorbable textile reinforcement properties of composite meshes. Descriptive comparison of values were obtained from benchtop testing of the textiles constitutive of the devices. Properties measured included: burst strength, resistance to plunger test, tear strength, suture pull-out strength (n=5 per group), and uniaxial breaking strength (n=6 per group). November 2016. 28. Based on NAMSA study #198929, Minimizing tissue attachment barrier performance, local tissue effects and tissue integration of Parietene™ DS composite mesh in a rat cecal abrasion model: occurrence rates of cecal soft tissue attachment to the mesh through macroscopic observations in the rat (n=18 test articles vs. n=18 Ethiocon ProceedTM* Surgical Mesh; p<0.05). October 2016. 29. Based on NAMSA study #194092, Pilot in vivo study: Parietene™ DS composite mesh versus competitive product in intraperitoneal pig model. Based on macroscopic, histologic, and scanning electronic microscopic (SEM) observations at four weeks in a porcine intraperitoneal implantation model (B1:K6n=6). December 2016. 30. Based on NAMSA study #212466, Pilot in vivo study: Parietene™ DS composite mesh versus competitive product in intraperitoneal pig model. Based on macroscopic and histologic observations at 12 weeks in a porcine intraperitoneal implantation model (n=6). December 2016. 31. . Based on internal report #T2294CR179. Design validation of Parietene™ DS composite mesh compatibility with tacks. May 2016. 32. Based on internal report #T2294CR181, Design verification of mesh suture strength in warp and weft direction. June 2016. sus procedimientos de reparación de hernia. 33. Based on internally sponsored preclinical study report #0506-141814, Report 10 days and 4 weeks implantation study in a porcine model, March 2012, and report #0506-141814, Report 10 days and 4 weeks implantation study – amendment 1, June 2012. 34. Tollens T, Den Hondt M, Devroe K, et al. Retrospective analysis of umbilical, epigastric, and small incisional hernia repair using the Ventralex™* hernia patch. Hernia. 2011;15(5):531-540. 35. Muysoms FE, Bontinck J, Pletinckx P. Complications of mesh devices for intraperitoneal umbilical hernia repair: a word of caution. Hernia. 2011;15(4):463-468. 36. Berrevoet F, Van den Bossche B, de Baerdemaeker L, de Hemptinne B. Laparoscopia evaluation shows deficiencies in memory ring deployment during small ventral hernia repair. World J Surg. 2010;34(7):1710-1715. 37. Burger JW, Halm JA, Wiismuller AR, ten Raa S, Jeekel J. Evaluation of new prosthetic meshes for ventral hernia repair. Surg Endosc. 2006;20(8):1320-1325. 38. Based on internally sponsored preclinical study report #0506-140983, Evaluation of the local tissue effects and tissue attachment minimization of a Parietex™ composite ventral patch in a rat caecal abrasion model, January 2012, and internally sponsored preclinical study report #0506-140983, Evaluation of the local tissue effects and tissue attachment minimization of a Parietex™ composite ventral patch in a rat caecal abrasion model – amendment 1, February 2012. 39. 11/2021– US-WC-2100106–[WF#5406475] 40. Based on internal Force TriVerse™ device report obtained from Global Cognos (FY2011 to mid FY2020) and Business Objects (mid FY20). 41. as per internal test report #RE0068753, a quantitative comparison with conventional monopolar electrosurgery: monopolar "L" hooked monopolar drag force measurements. March 2017. 42. per internal test report #REQ0115057, monopolar performance of the Valleylab™ mode on the Valleylab™ FT10 energy platform. September 2017. 43. per internal memo #RE00105221, power equivalency memo for the Valleylab™ FX8 and FT10 power platforms (VLFX8GEN/VLFT10GEN). July 2017. 44. Based on internal test report #RE00329878 rev A, Marketing evaluation of surgeon experience using the Sonicision™ 7 cordless ultrasonic curved jaw dissector. April 14-15 and 20-22, 2021. 45. Ding, Z., Wable, G., Rane, A. Use of LigaSure bipolar diathermy system in vaginal hysterectomy. J Obstet Gynaecol. 2005;25(1): 49-51. 46. Levy, B., Emery, L., Randomized trial of suture versus electrosurgical bipolar vessel sealing in vaginal hysterectomy. Obstet Gynecol. 2003;102(1):147-151. 47. Targarona, et al. A prospective randomized comparison of conventional electrosurgery, biopolar computer-controlled electrosurgery and ultrasonic dissection. Operative Outcome and Cost analysis. Surgical Innovation. Dic. 2005;12(4):339-344. 48. Manouras, et al. Sutureless open low anterior resection with total mesorectal excision for rectal cancer with the use of the electrothermal bipolar vessel sealing system. 2007. Med Sci Monit; 13(5): CR224-230. 49. Tamussino, K., et al. Electrosurgical bipolar vessel sealing for radical abdominal hysterectomy. Gynecologic Oncology. Feb. 2005; 96(2):320-322. 50. Leonardo, C., et al. Laparoscopic nephrectomy using LigaSure system: preliminary experience. Journal of Endourology. Oct. 2005;19(8):976-8. 51. Daskalopoulos, G., et al. Electrothermal bipolar coagulation for radical prostatectomies and cystectomies: a preliminary case-controlled study. International Urology and Nephrology. 2004;36(2):181-185.52. Cronje, H.S., et al. Electrosurgical bipolar vessel sealing during vaginal hysterectomy. Int J Gynaecol Obstet. Dic. 2005;91(3):243-5. 53. Araki, Y., et al. Clipless hand-assisted laparoscopic total colectomy using LigaSure Atlas. Kurume Medical Journal. 2004;51(2):105-8. 54. Campagnacci, R., et al. Electrothermal bipolar vessel sealing device vs. ultrasonic coagulating shears in laparoscopic colectomies: a comparative study. Surg Endosc. 8 feb. 2007.55. Takada et al. Comparative study of electrothermal bipolar vessel sealer and ultrasonic coagulating shears in laparoscopic colectomy. Surgical Endoscopy. 2005;19:226-228. 56. Demirturk, F., et al. Comparison of the use of electrothermal bipolar vessel sealer with harmonic scalpel in total laparoscopic hysterectomy. J Obst Gynaecol Res. 2007; 33 (3):341-345. 57. 11/2021– US-WC-2100106–[WF#5406475]
Nº anvisa: 10349000691, 10349000279, 10349000284, 10349000284, 10349000038, 10349000196, 80052020066, 10349000305, 10349000306, 10349000419, 10349000430, 10349000431, 10349000239, 10349000228, 10349000501, 10349000502, 10349000505, 10349000523, 10349000849, 10349009018, 10349009021, 10349009020, 10349000506, 10349000523, 10349000439, 10349001001, 10349000262, 10349000531, 10349000537, 10349000538, 10349000539, 10349000540, 10349000527, 10349000690, 10349000514, 10349000239, 80052020055, 10349000305, 10349000306, 10349000419, 10349000430, 10349000431, 10349000305, 10349000306, 10349000419, 10349000430, 10349000431, 10349000269, 10349000535, 10349000691, 10349000867, 10349000583, 10349000535, 10349001256, 10349000584, 10349000684, 10349001285, 10349001289, 10349000305, 10349000306, 10349000419, 10349000430, 10349000431.
CONTEÚDO APENAS PARA PROFISSIONAIS DE SAÚDE. ©2023 Medtronic. Todos os direitos reservados. Medtronic, o logotipo da Medtronic e Outros são, coletivamente, marcas comerciais da Medtronic. Todas as outras marcas são marcas comerciais de uma empresa da Medtronic. A Covidien é uma empresa que faz parte do grupo Medtronic.


