ILLUMISITE™ fluoroscopic navigation platform
The lungs are dynamic.
So is our imaging.
The leading electromagnetic lung navigation platform that corrects for CT-to-body divergence.1,2

Don’t let lung motion put biopsy at a standstill.
The lungs are always in motion. To move lung cancer diagnosis forward, technology must be as dynamic as the lungs themselves.
That’s why you need a lung navigation platform that corrects for CT-to-body divergence.
The ILLUMISITE™ platform corrects for CT-to-body divergence — the discrepancy between a static CT scan and the breathing lung — with real-time visualization that enhances image clarity, and adjusts to a dynamic environment.3-5,†,‡ Now it’s compatible with digital fluoroscopes, delivering expanded fluoroscopic capability during lung biopsy procedures.
Successfully navigate to the nodule, correct for CT-to-body divergence, and visualize and maintain alignment with one fully integrated navigational platform.4-6,†
With the ILLUMISITE™ platform, it’s all possible.
†Based on evidence from a single-center prospective study including a total of 82 consecutive patients
‡ Based on evidence from a single-center retrospective study including a total of 72 consecutive patients.
See the real target.
Accuracy is no longer a moving target with the ILLUMISITE™ platform’s fluoroscopic navigation technology.
Clinically proven to help you accurately navigate to lung nodules with its unique fluoroscopic navigation technology utilizing digital tomosynthesis.1-4,†,‡ the ILLUMISITE™ platform is designed to provide the kind of diagnostic accuracy you rely on.
CT-to-body divergence can be caused by:
- Static CT image in dynamic lung environment1,5
- Changes from anesthesia1,5
- Tissue distortion due to robotic and flexible scopes1,5
Fluoroscopic navigation technology corrects CT-to-body divergence by:
- Visually enhancing nodules7
- Allowing alignment of the catheter to the nodule throughout the procedure4,†
- Pinpointing the correct location before a biopsy4,5,†
†Based on evidence from a single-center prospective study including a total of 82 consecutive patients. ‡ Based on evidence from a single-center retrospective study including a total of 72 consecutive patients.
Lock on in real time.
At every stage of the biopsy procedure, you’ll remain on target with ongoing guidance.
With the ILLUMISITE™ platform, the precision of continuous guidance makes continued target alignment possible across multiple tool uses — without the need to reinsert the locatable guide.4,†
And it’s designed for multidirectional sampling for a more thorough biopsy.
†Based on evidence from a single-center prospective study including a total of 82 consecutive patients

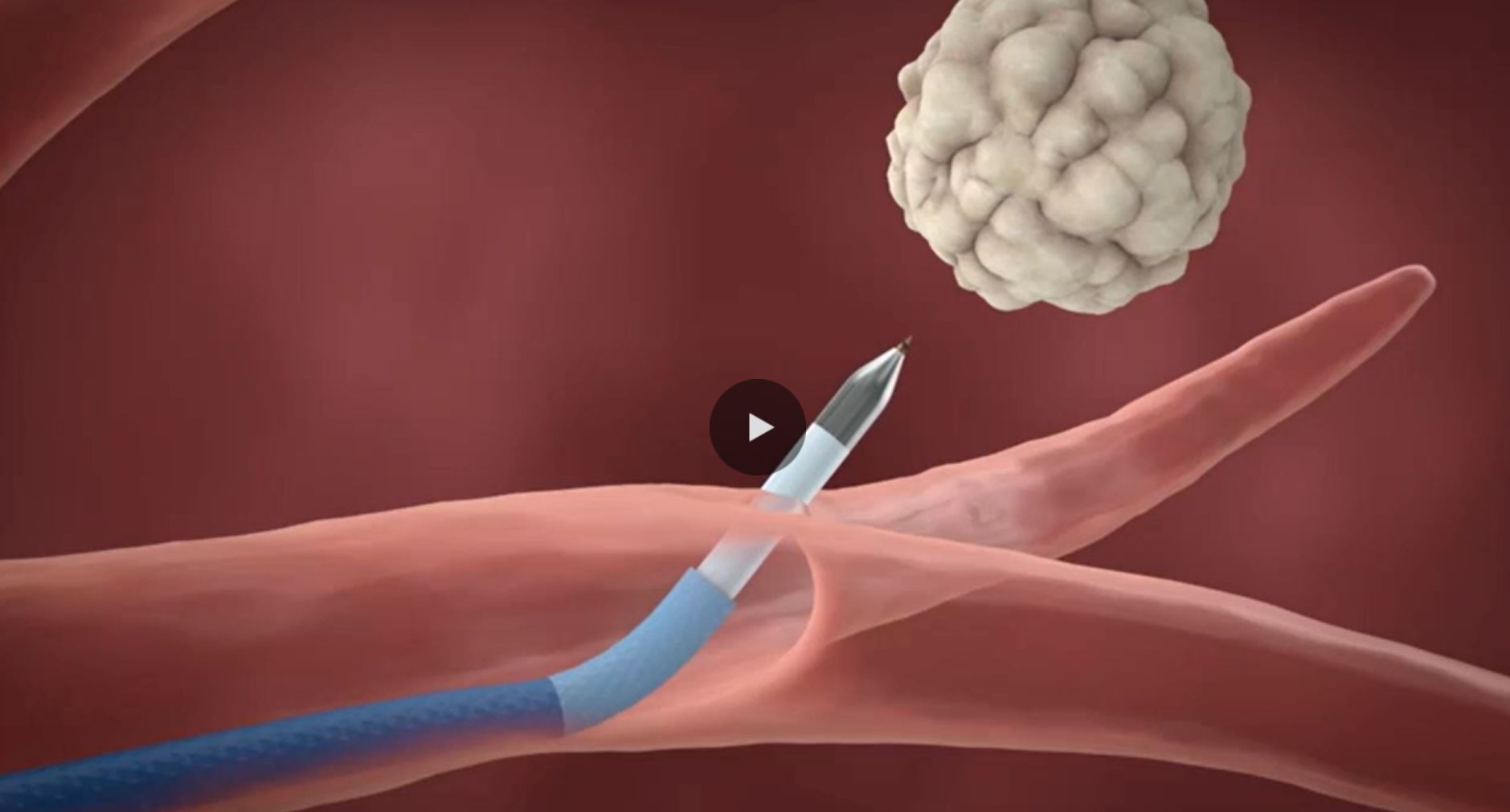
Travel outside the airways.
New possibilities for lung disease detection and management are within reach when you can extend the reach of bronchoscopy.
Locate hard-to-reach lesions — especially outside of visible airways6 — with the CrossCountry™ transbronchial access tool.†,‡
†For more information regarding the CrossCountry™ transbronchial access tool, please contact your account manager or clinical education specialist.
‡Based on NAVIGATE study: a large prospective observational multicenter multinational study of 1388 subjects (NAVIGATE).
Digital compatibility that moves lung biopsy forward.
The ILLUMISITE™ platform is now compatible with digital fluoroscopes, delivering expanded fluoroscopic capability, for new possibilities. This is device innovation that promotes operational efficiency to facilitate diagnosis and treatment.
- Imaging compatibility powers access.
- The ILLUMISITE™ platform gives you new ways to expand your imaging compatibility with digital fluoroscopy. Increasing your access to fluoroscopic navigation technology, which uses digital tomosynthesis, means more certainty that you are in the right location before you biopsy.1-4,†
- Integrated imaging − for accuracy you can rely on.
- Achieving the navigation accuracy you need to reach the target nodule requires high-quality, real-time imaging. The ILLUMISITE™ platform features integrated imaging that is clinically proven to accurately navigate to lung nodules1-4,†,‡ and correct for CT-to-body divergence.1-4,†,‡
- Maximize your imaging investment.
- Explore how expanded fluoroscopic capability may amplify the clinical and economic value of your imaging investment. With digital compatibility, you can unlock the full potential of the ILLUMISITE™ platform — enabling CT-to-body divergence correction.1-4,†,‡
†Based on evidence from a single-center prospective study including a total of 82 consecutive patients.
‡ Based on evidence from a single-center retrospective study including a total of 72 consecutive patients.

Catalyze timely identification and care coordination.
Imagine how different the outlook for patients could be if you could identify, follow, and manage care for lung nodule patients.
The LungGPS™ patient management platform† is designed to help provide continuity in the patient journey − spanning lung screening, incidental nodule management, and thoracic tumor board collaboration.
Our comprehensive imaging and data software delivers an all-in-one solution with a multidisciplinary approach.
†The LungGPS™ platform is an information management software only and does not diagnose or treat lung cancer nor replace lung cancer assessment in standard clinical practice. The LungGPS™ platform should be used in conjunction with professional guidelines for patient management decision. The LungGPS ™ platform is only available for sale in the United States.
Tools that take aim at lung challenges
Drive confidence with our portfolio of lung biopsy tools.

Sensing catheters
A sensor coil embedded in the distal tip generates continuous positional data, so you can maintain alignment on the target even after the locatable guide is removed.
Arcpoint™ pulmonary needle
Features a braided sheath for trackability and maneuverability tapered for ease of sampling. The short, rigid metal needle maximizes flexibility. There is also an optional stylet for additional rigidity if needed.

GenCut™ core biopsy system
Using a proprietary blade design, this tool helps you obtain core tissue samples for molecular genetic analysis and enables continuous sampling.
SuperLock™ nitinol coil fiducial markers
Features a braided sheath for trackability and maneuverability tapered for ease of sampling. The short, rigid metal needle maximizes flexibility. There is also an optional stylet for additional rigidity if needed.

What physicians see
Physicians are watching the benefits of the ILLUMISITE™ fluoroscopic navigation platform play out at their institutions every day.
A new movement for lung cancer care
Staying one move ahead of lung cancer takes innovative surgical products, minimally invasive techniques, and continuous collaboration.
Explore how the Medtronic Lung Health Program is dedicated to advancing lung health. All focused on our vision to transform lung cancer from a terminal illness into a manageable and potentially curable condition.

Lung health webinar series
Led by world-renowned key opinion leaders, our monthly series offers a forum to keep up with important topics in lung care. Join the conversation, and watch our past events.
- Accuracy is everything: recent clinical evidence in navigations
- Biopsy with confidence: correcting for CT-to-body divergence
- Optimizing lung cancer care: building a lung health program
- Innovation for optimized procedures: visualization and biopsy techniques
- Dye marking and fiducial placement: enhance your visualization experience
Your next move
Get the information you need for the lung health results you want.
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic
Indications for use
ILLUMISITE™ fluoroscopic navigation platform: The ILLUMISITE™ platform is indicated for displaying images of the tracheobronchial tree to aid the physician in guiding endoscopic tools or catheters in the pulmonary tract and to enable marker placement within soft lung tissue. It does not make a diagnosis and is not an endoscopic tool. Not for pediatric use.
WARNING: The ILLUMISITE™ platform may only be used by a qualified bronchoscopist.
CONTRAINDICATIONS:
Flexible bronchoscopy should be performed only when the relative benefits outweigh the risks. Absolute contraindications include, but are not limited to:
• Absence of consent from the patient or his /her representative, unless a medical emergency exists and the patient is not competent to give consent.
• Lack of adequate facilities and personnel to care for emergencies such as cardiopulmonary arrest, pneumothorax, or bleeding.
• Inability to adequately oxygenate the patient during the procedure.
• For large patients, inability to place all three patient sensors within the sensing volume.
The danger of a serious complication from bronchoscopy is especially high in patients with the disorders listed below. These conditions are usually considered absolute contraindications, unless risk-benefit assessment warrants the procedure:
• Coagulopathy or bleeding diathesis that cannot be corrected.
• Severe obstructive airways disease.
• Severe refractory hypoxemia.
• Unstable hemodynamic status including dysrhythmias.
Relative contraindications or conditions involving increased risk for Fiber-optic Bronchoscopy in adults include but are not limited to:
• Recent myocardial infarction or unstable angina.
• Uremia and pulmonary hypertension (possibility of serious hemorrhage after biopsy).
• Lung abscess (danger of flooding the airway with purulent material).
• Respiratory failure requiring mechanical ventilation.
• Known or suspected pregnancy (because of radiation exposure).
CrossCountry™ transbronchial access tool
The CrossCountry™ transbronchial access tool is to be utilized through a flexible endoscope with an extended working channel by physicians who are trained in endoscopic techniques to puncture the tracheobronchial wall and facilitate access of additional endobronchial tools for patients with endobronchial lesions, peripheral lung nodules, or lung masses.
Always refer to the instructions for use included with the product for complete indications, contraindications, warnings, and precautions.
LungGPS™ patient management platform
Intended Use:
The Lung Cancer Screening Manager is an administrative software tool for use by a patient navigator intended to simplify adherence to hospital-defined administrative protocols. The software is a workflow tool to aid the patient coordinator in their daily tasks to manage a lung screening program. The Lung Cancer Screening Manager will query healthcare information systems (HIS, RIS, EMR, PACS) for data on the status of lung screening participants and proactive patient monitoring. The application will not be involved in the diagnosis, prevention, or treatment of disease.
The Incidental Nodule Manager is intended to aid in the retrieval and review of patient data and automate routine administrative and instructive tasks based on pre-established workflow protocols (e.g., Fleischner Society Criteria) and to provide configuration options to meet specific-site needs. The application communicates with ancillary image and non-image-based information systems through industry-standard interfaces and provides administrators and healthcare professionals with patient status throughout the care continuum. The software also provides aggregate data and key performance indicators (KPIs) on various pre-established clinical program parameters.
Multidisciplinary Team Orchestrator is intended to bring together relevant patient data and the multidisciplinary team to enable collaborative decision making. It streamlines preparation, enhances review and analysis, and empowers the cancer care team to reach clinical treatment decisions based upon rich dashboards, diagnostic images, reports, and structured patient data. The sessions can be attended virtually or in-person — synchronously or asynchronously.
Philips DynaCAD™* Lung Screening Software is indicated for use as a general imaging workstation. It is intended to be used to acquire, store, transmit and display images from medical scanning devices. Specific indications for use for the software are the display of a composite view of 2D cross-sections, and 3D volumes of chest CT images, including findings or regions of interest (ROI) identified or Computer Assisted Detection (CAD) findings.
CONTRAINDICATIONS:
The LungGPS™ platform is intended to integrate with specific file output of sequencing instruments such as Illumina sequencers as well as integrate with specific electronic medical records and external database warehouses. The system should not be used with any instruments that have not been tested and verified as compatible with the LungGPS™ platform.
The LungGPS™ patient management platform is commercialized in partnership with Philips.
1. Pritchett MA, Bhadra K, Calcutt M, Folch E. Virtual or reality: divergence between preprocedural computed tomography scans and lung anatomy during guided bronchoscopy. J Thorac Dis. 2020;12(4):1,595–1,611.
2. Medtronic financial and market analysis as of November 2021 of ENB and robotic lung navigation systems in market demonstrating Medtronic leading market share as defined by percentage of capital system sales.
3. Dunn BK, Blaj M, Stahl J, Speicher J, Anciano C, Hudson S, Kragel EA, Bowling MR. Evaluation of electromagnetic navigational bronchoscopy using tomosynthesis-assisted visualization, intraprocedural positional correction and continuous guidance for evaluation of peripheral pulmonary nodules. J Bronchology Interv Pulmonol. 2022.
4. Avasarala SK, Roller L, Katsis J, et al. Sight unseen: diagnostic yield and safety outcomes of a novel multimodality navigation bronchoscopy platform with real-time target acquisition. Respiration. 2022;101(2):166–173.
5. Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic navigation bronchoscopy with tomosynthesis-based visualization and positional correction: three-dimensional accuracy as confirmed by cone-beam computed tomography. J Bronchology Interv Pulmonol. 2021; 28(1):10–20.
6. Folch EE, Pritchett MA, Nead MA, et al. Electromagnetic navigation bronchoscopy for peripheral pulmonary lesions: one-year results of the prospective, multicenter NAVIGATE study. J Thorac Oncol. 2019;14(3): 445–458.
7. Based on internal report #DGR00452 for the ILLUMISITE™ platform. Aug. 2017.
©2023 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 02/2023 - US-RE-2300128