

It doesn’t stop even when healthcare as we know it changes forever. It moves swiftly — and relentlessly.
We have to move faster.
Because of COVID-19, the world has seen a dramatic decrease in proactive cancer screenings.1 This puts patients at higher risk for late-stage detection, limiting their treatment options and drastically reducing survival rates.2,3 That’s why it’s important to get patients back into the care continuum — and push them toward early detection.
How?
It starts with a conversation. Our Lung Cancer Toolkit was designed to help you share the facts with your patients and encourage them to get screenings — before it’s too late.
Because when it comes to lung cancer, time saves lives. The more time you give your patients, the more treatment options they have. And when you and your team are empowered to catch nodules early — we can turn this devastating diagnosis into a manageable condition.
of lung cancer patients are diagnosed at stages III & IV.
Average survival rate at five years is
When diagnosed and treated early, survival rate jumps to
of actionable incidental nodules are malignant.
More than
of incidental nodules go unaddressed.
costlier to treat stage IV lung cancer than stage I lung cancer.
†Based on 10-year survival after diagnosis and resection within one month.
‡ The average cost to treat a patient with stage IV lung cancer is $21,441, compared to $7,239 for a patient with stage I lung cancer.
Transforming lung cancer care is no easy task. It requires unprecedented collaboration and planning — and there’s no better time to start than now.
To help identify and treat patients earlier, a number of steps have to be taken:

Let’s work together to develop a Lung Health initiative.

Get the right tools and technology so you’re ready to move fast.
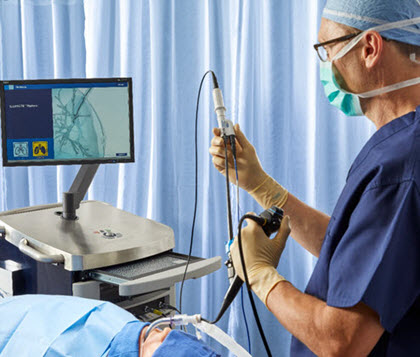
Identify, manage, and treat patients earlier.

Build a program, get training, access resources, and rely on ongoing support.
Talk to your patients. Download the toolkit now.
When it comes to lung cancer, time saves lives. The more time you give your patients, the more treatment options they have.
See how one patient’s early diagnosis of an incidental nodule led to swift treatment and a positive outcome.
With our comprehensive lung health portfolio.
Fluoroscopic navigation
technology results in a
POINT INCREASE
in diagnostic yields
§25 percentage point increase using fluoroscopic navigation (79%, 53/67) versus standard navigation (54%, 55/101) in a single-center retrospective design.
Fluoroscopic navigation can help correct CT-to-body divergence,7,8 enhancing your ability to accurately navigate and biopsy the lung.8,9 And the easier it is to identify malignant nodules, the earlier you can begin treatment.
Learn how the ILLUMISITE™ platform is transforming lung care.
Nearly
new lung cancer cases in 2020.
Lung nodule patients need to be identified early, monitored closely, and managed with the highest level of care. But disparate healthcare systems leave gaps in the patient care continuum that can put these patients at risk. Until now.
The LungGPS™ platform is an all-in-one solution designed to streamline lung patient management.

Today, 71 percent of patients with incidental lung nodules don’t receive follow-up care.5 This means that countless lung cancer cases are left untreated until it’s too late. Together, we can change these numbers and give patients the care — and time — they deserve.
DOWNLOAD OUR TOOLKITTo learn more about our lung care solutions, please visit our website at medtronic.com.
ILLUMISITE™ Platform: The ILLUMISITE™ Platform is indicated for displaying images of the tracheobronchial tree to aid the physician in guiding endoscopic tools or catheters in the pulmonary tract and to enable marker placement within soft lung tissue. It does not make a diagnosis and is not an endoscopic tool. Not for pediatric use.
WARNING: The ILLUMISITE™ platform may only be used by a qualified bronchoscopist.
CONTRAINDICATIONS:
Flexible bronchoscopy should be performed only when the relative benefits outweigh the risks. Absolute
contraindications include, but are not limited to:
- Absence of consent from the patient or his/her representative, unless a medical emergency exists and the patient is not competent to give consent.
- Lack of adequate facilities and personnel to care for emergencies such as cardiopulmonary arrest, pneumothorax, or bleeding.
- Inability to adequately oxygenate the patient during the procedure.
- For large patients, inability to place all three patient sensors within the sensing volume.
The danger of a serious complication from bronchoscopy is especially high in patients with the disorders listed below.
These conditions are usually considered absolute contraindications, unless risk-benefit assessment warrants the procedure:
- Coagulopathy or bleeding diathesis that cannot be corrected.
- Severe obstructive airways disease.
- Severe refractory hypoxemia.
- Unstable hemodynamic status including dysrhythmias.
Relative contraindications or conditions involving increased risk for Fiber-optic Bronchoscopy in adults include but are not limited to:
- Recent myocardial infarction or unstable angina.
- Uremia and pulmonary hypertension (possibility of serious hemorrhage after biopsy).
- Lung abscess (danger of flooding the airway with purulent material).
- Respiratory failure requiring mechanical ventilation.
- Known or suspected pregnancy (because of radiation exposure).
LungGPS™ Patient Management Platform: The LungGPS™ platform is an information management software only and does not diagnose or treat lung cancer nor replace lung cancer assessment in standard clinical practice. The LungGPS™ platform should be used in conjunction with professional guidelines for patient management decision.
CONTRAINDICATIONS: The LungGPS™ platform is intended to integrate with specific file output of sequencing instruments such as Illumina sequencers as well as integrate with specific electronic medical records and external database warehouses. The system should not be used with any instruments that have not been tested and verified as compatible with the LungGPS™ platform.
The LungGPS™ patient management platform is commercialized in partnership with Philips.
1. Alkatout I, Biebl M, Momenimovahed Z, et al. Has COVID-19 Affected Cancer Screening Programs? A Systematic Review. Front Oncol. 2021;11:675038. 2021.
2. Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021 [published correction appears in CA Cancer J Clin. 2021 Jul;71(4):359]. CA Cancer J Clin. 2021;71(1):7-33. doi:10.3322/caac.21654
3. Henschke CI, Yankelevitz DF, Libby DM, Pasmantier MW, Smith JP, Miettinen OS. Survival of patients with stage I lung cancer detected on CT screening. N Engl J Med. 2006;355(17):1763–1771.
4. Gildea TR, DaCosta Byfield S, Hogarth DK, Wilson DS, Quinn CC. A retrospective analysis of delays in the diagnosis of lung cancer and associated costs. Clinicoecon Outcomes Res. 2017;9:261–269.
5. Tanner NT, Aggarwal J, Gould MK, et al. Management of pulmonary nodules by community pulmonologists: a multicenter observational study. Chest. 2015;148(6):1405–1414.
6. Blagev DP, Lloyd JF, Conner K, et al. Follow-up of incidental pulmonary nodules and the radiology report. J Am Coll Radiol. 2014;11(4):378–383.
7. Pritchett MA, Bhadra K, Mattingley JS. Electromagnetic Navigation Bronchoscopy With Tomosynthesis-based Visualization and Positional Correction: Three-dimensional Accuracy as Confirmed by Cone-Beam Computed Tomography. J Bronchology Interv Pulmonol. 2021;28(1):10-20. doi:10.1097/LBR.0000000000000687.
8. Based on internal report #DGR00452, FluoroSync verification test characterization. August 2017.
9. Based on internal report #DGR00512, Comparison of lesion visibility in a 3-D fluoroscopic visualization image vs. a standard fluoroscopic image. March 2018.
10. Aboudara M, Roller L, Rickman O, et al. Improved diagnostic yield for lung nodules with digital tomosynthesis-corrected navigational bronchoscopy: Initial experience with a novel adjunct. Respirology. 2020;25(2):206-213. doi:10.1111/ resp.13609.
© 2021 Medtronic. All rights reserved. Medtronic, Medtronic logo and Further, Together are trademarks of Medtronic. All other brands are trademarks of a Medtronic company. 09/2021-US-RE-2100539 [WF#5398660]