

Nellcor™ Pulse Oximetry
Neonatal Sensors
Specially designed for neonatal monitoring.
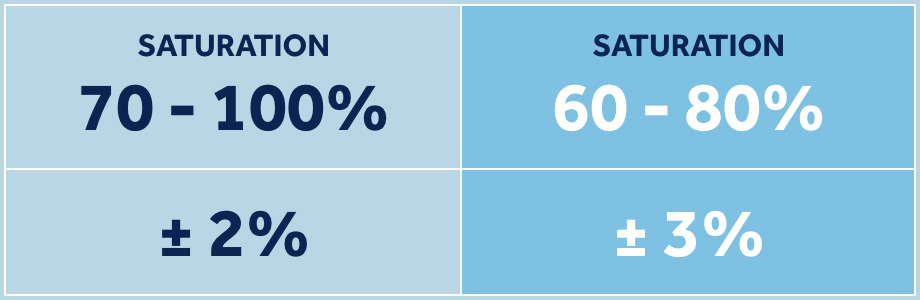
Request a Sample KitYou face unique challenges and need reliable data. Our neonatal sensors are tested under real patient conditions to be accurate, even through motion and low saturation.† Nellcor™ pulse oximetry with OxiMax™ technology has SpO2 accuracy of ±2% in the standard range of 70% to 100% saturation and LoSat SpO2 accuracy of ±3% in the range of 60% to 80% saturation, enabling clinicians to accurately assess patients’ oxygen saturation.1
A vaginal birth baby will not reach 70% SpO2 until the third minute of life, a c-section baby until the fourth minute of life.2 And many conditions can lead to hypoxia, especially in premature babies. Because of these poor perfusion situations, you need accurate data to provide the most appropriate care.
MaxN SensorsThe removal of adhesives can damage the fragile skin of premature neonates and potentially lead to infection.3 To limit this risk, we developed the Nellcor™ SpO2 nonadhesive sensor for neonatal patients.
Nonadhesive Sensors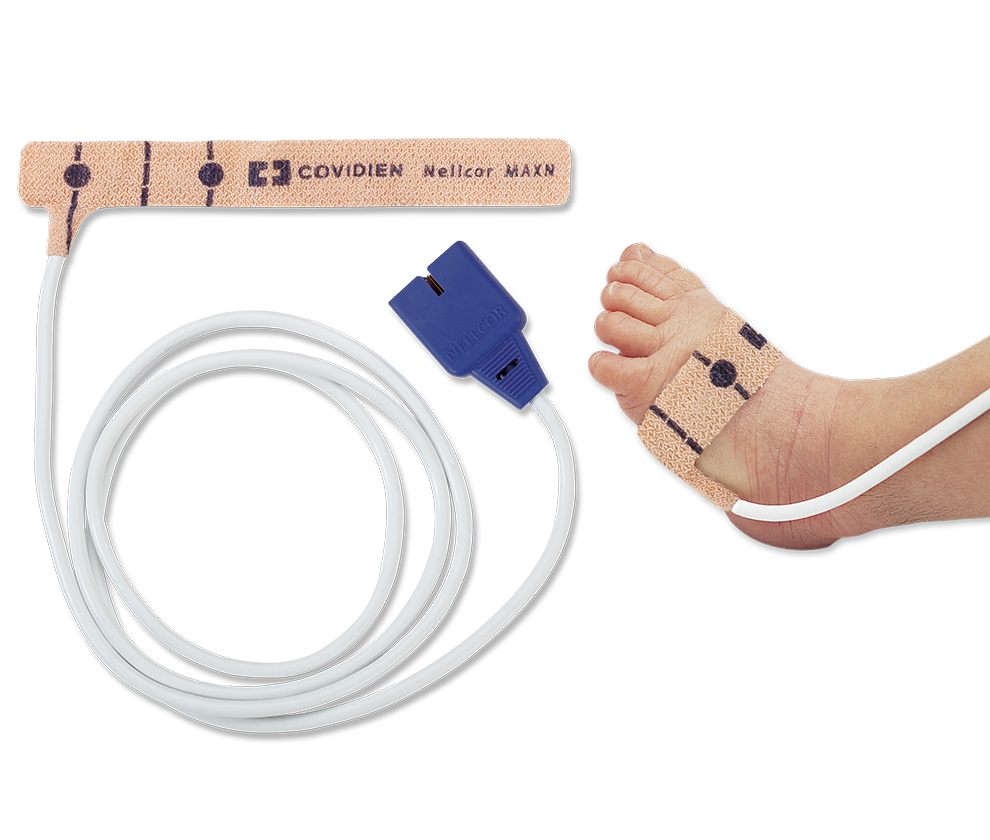
Nellcor™ pulse oximetry MAXN sensors are specifically designed for for the challenges of neonates.
Offers industry-leading SpO2 accuracy during low saturation conditions, of ±3% within the range of 60% to 80% saturation.4
Helps enable informed clinical decisions for patients in low saturation range, including newborns, pre-surgery CHD patients, and neonates experiencing apneas and bradys.
The sensors are designed for sterile, single-patient use, offering infection-control advantages.
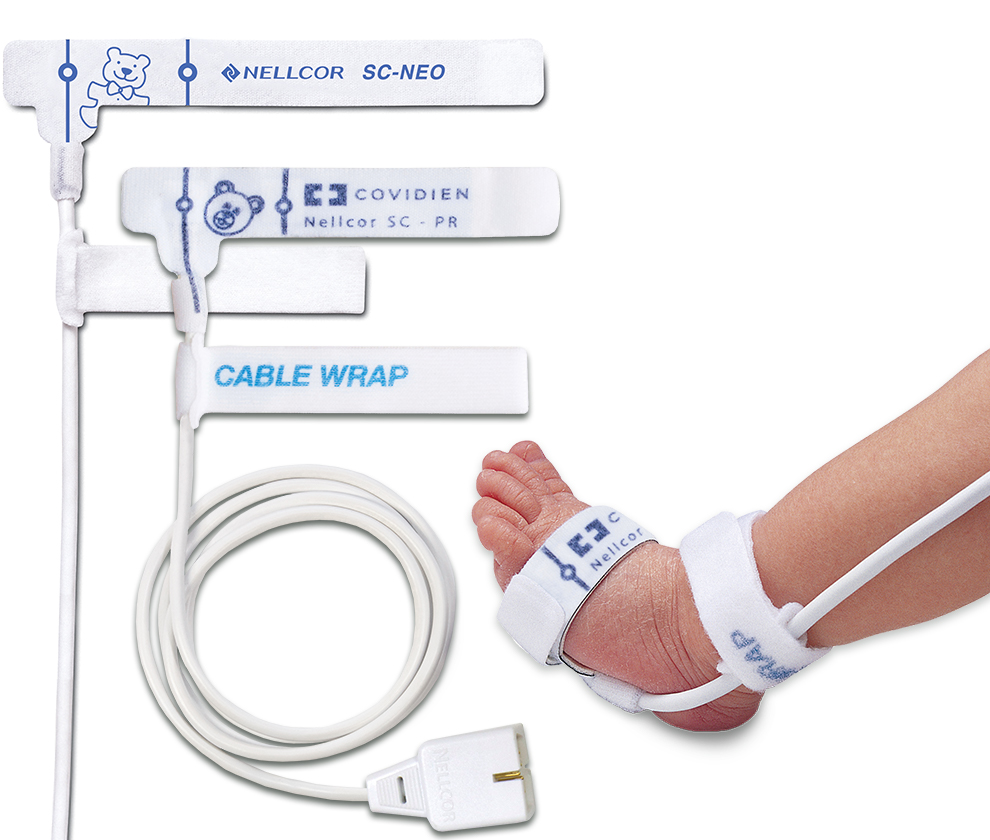
Nellcor™ pulse oximetry nonadhesive sensors are made of a soft, pliable, low-profile foam material that give them "stiction" to help keep the sensor in place without adhesives. The sensors fasten easily with a small hook and loop strap, giving a customizable fit.
A comfortable, secure, second-skin fit keeps the sensor in place to promote reliable readings.
The sensors offer a high degree of accuracy comparable to adhesive digit sensors even for patients who are poorly perfused or moving.†
The sensors are designed for sterile, single-patient use, offering infection-control advantages.
† Oxygen saturation accuracy can be affected by certain environmental, equipment, and patient physiologic conditions (as discussed in the operator’s manual for the monitor) that influence readings of SpO2. Please consult the IFU and manual for full safety information.
The Nellcor™ pulse oximetry monitoring system should not be used as the sole basis for diagnosis or therapy and is intended only as an adjunct in patient assessment.
The best way to see the gentle design of our nonadhesive sensor is to see it live. Request a complimentary sensor sample kit today by completing the form below.
The size, stability, and values of the company behind the products make a crucial difference to you and your patients. Our mission guides everything we do, including the meticulous standards-based research, development, and testing for which we’re known.
1. PMB10N (LP100):10143258 - Clinical Report, OxiCable (LP100 embedded) Abbreviated Sensor Line including the validation at 70%-100% saturation range with MaxA, MaxFAST, SCA, OxicliqA, DS100A, DYS-E
-RE00105732, SpO2 Accuracy Clinical Report of USB Pulse Oximetry Monitor Interface Cable with FlexMax Reusable SpO2 Sensor via Reference CO-OximetryRE00085099 Verification Test Report, Sensor Accuracy, Oxicable including simulated patient data
-NELL1: 10011350 (N600x) - Clinical Summary Report for N600x including the following validation studies: 1) N600x performance with MaxA, MaxFast, SC-A, DS-100A, OxiCliq A, D-YSE at 70-100% saturation range and 2) N600x comparision to N595/MaxA.
-10028895 - MP100_O6 (Nell 1 equivalent) - Clinical Summary Report including validation of accuracy of MP100-O6 with Max AL, DS100A, D-YSE, OxiCliq, SC-A and MaxFast.
2. Rabi Y, Dawson JA. Oxygen therapy and oximetry in the delivery room. Seminars in Fetal & Neonatal Medicine. 2013.
3. Widiati E, Nurhaeni N, Gayatri D. Medical-device related pressure injuries in the delivery room. Seminars in Fetal & Neonatal Medicine. 2013.
4. -10011350 (N600x) - Clinical Summary Report for N600x including the following validation studies: 1) N600x performance with MaxA, MaxFast, SC-A, DS-100A, OxiCliq A, D-YSE at 70-100% saturation range and 2) N600x comparision to N595/MaxA.
-10028895 - MP100_O6 (Nell 1 equivalent) - Clinical Summary Report including validation of accuracy of MP100-O6 with Max AL, DS100A, D-YSE, OxiCliq, SC-A and MaxFast.