

Mechanical ventilation remote access
Omnitool software provides clinicians with remote access to the Puritan Bennett™ 980 ventilator. Using Omnitool software allows clinicians to remotely view and adjust the ventilator settings outside of a patient room.
Complete this form and your local field service engineer will respond to help with your request.
We all are facing an unprecedented challenge. The medical and healthcare communities in the United States and across the world are responding to a medical emergency involving spread of the Novel Coronavirus Disease (“COVID-19”). On January 30, 2020, the World Health Organization (“WHO”) declared the outbreak of COVID-19 a Public Health Emergency of International Concern (“PHEIC”), and, on March 11, 2020, the WHO Director General declared COVID-19 a pandemic. On March 13, 2020, President Donald J. Trump declared a National Emergency in the United States due to the COVID-19 outbreak.
In response to this crisis, Medtronic is working around the clock to create new solutions for healthcare providers. To this end, and in support of the public health and medical response of governmental agencies around the world, Medtronic is releasing this remote access software to help doctors and patients dealing with COVID-19.
To request installation of the software, please register here. Note that the software is provided subject to the terms of the Software License Agreement linked on this page
Find the BDU ventilator Serial Number on the back of your Puritan Bennett™ 980 ventilator:
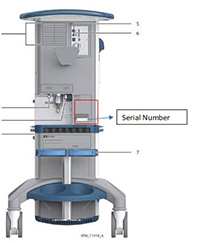
Or, find the BDU ventilator Serial Number on screen:
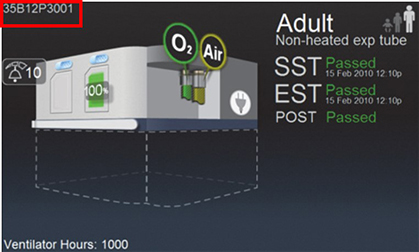
† This feature was developed in accordance with the US FDA Enforcement Policy for Ventilators and Accessories and Other Respiratory Devices During the Coronavirus 2019 (COVID-19) Public Health Emergency. This feature has not been cleared by the US FDA