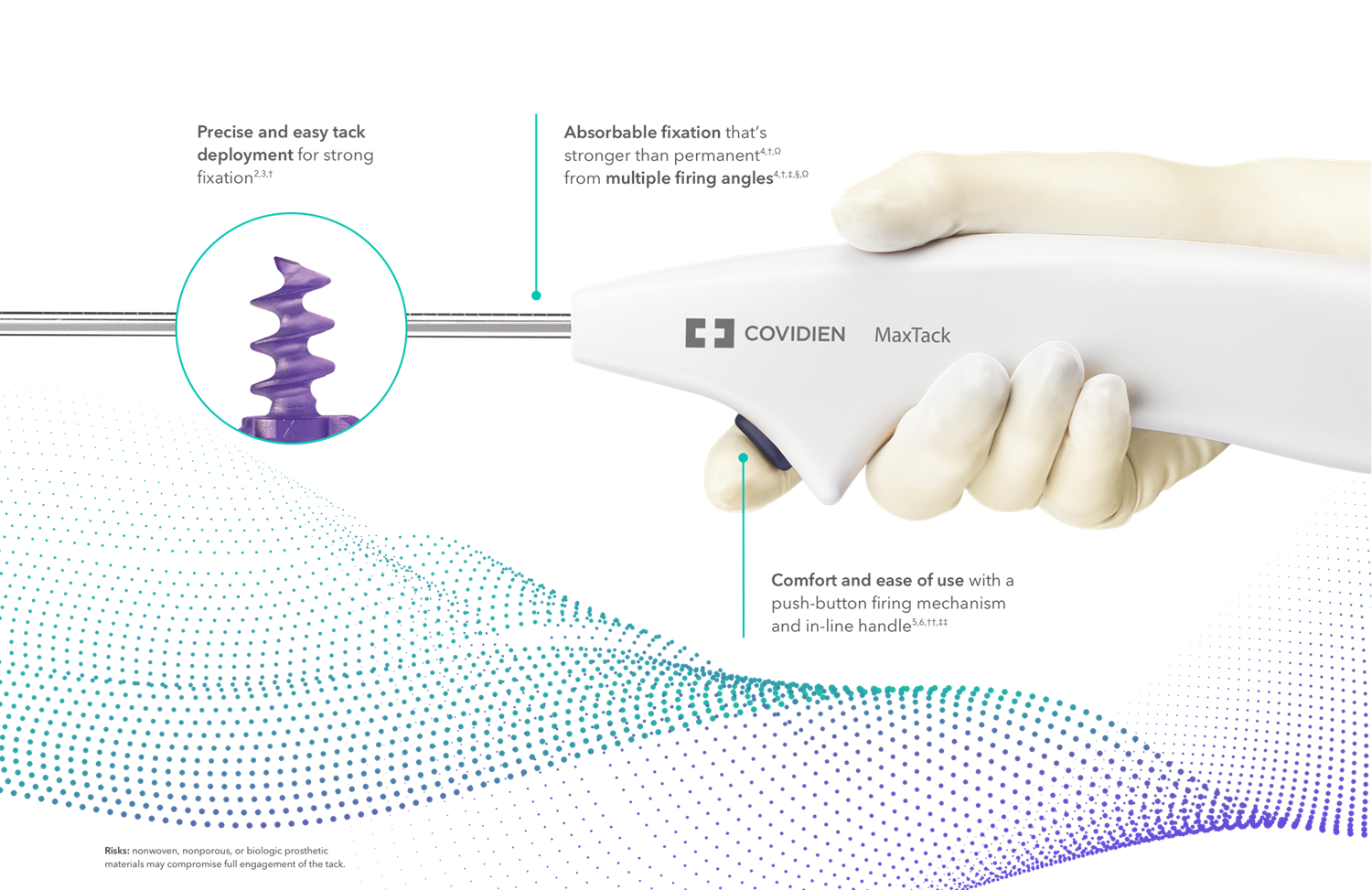
*Taking pre-launch orders: 510k cleared.
†Based on benchtop data, not necessarily indicative of human clinical outcomes.
‡Compared to competitive Sorbafx™*, Optifx™*, Securestrap™*, Capsure™*, and Medtronic absorbable tackers AbsorbaTack™, Reliatack™ devices.
§Compared to competitive straight-tacker and articulated-tacker Optifx™* AT and Medtronic articulated-tacker ReliaTack™ devices.
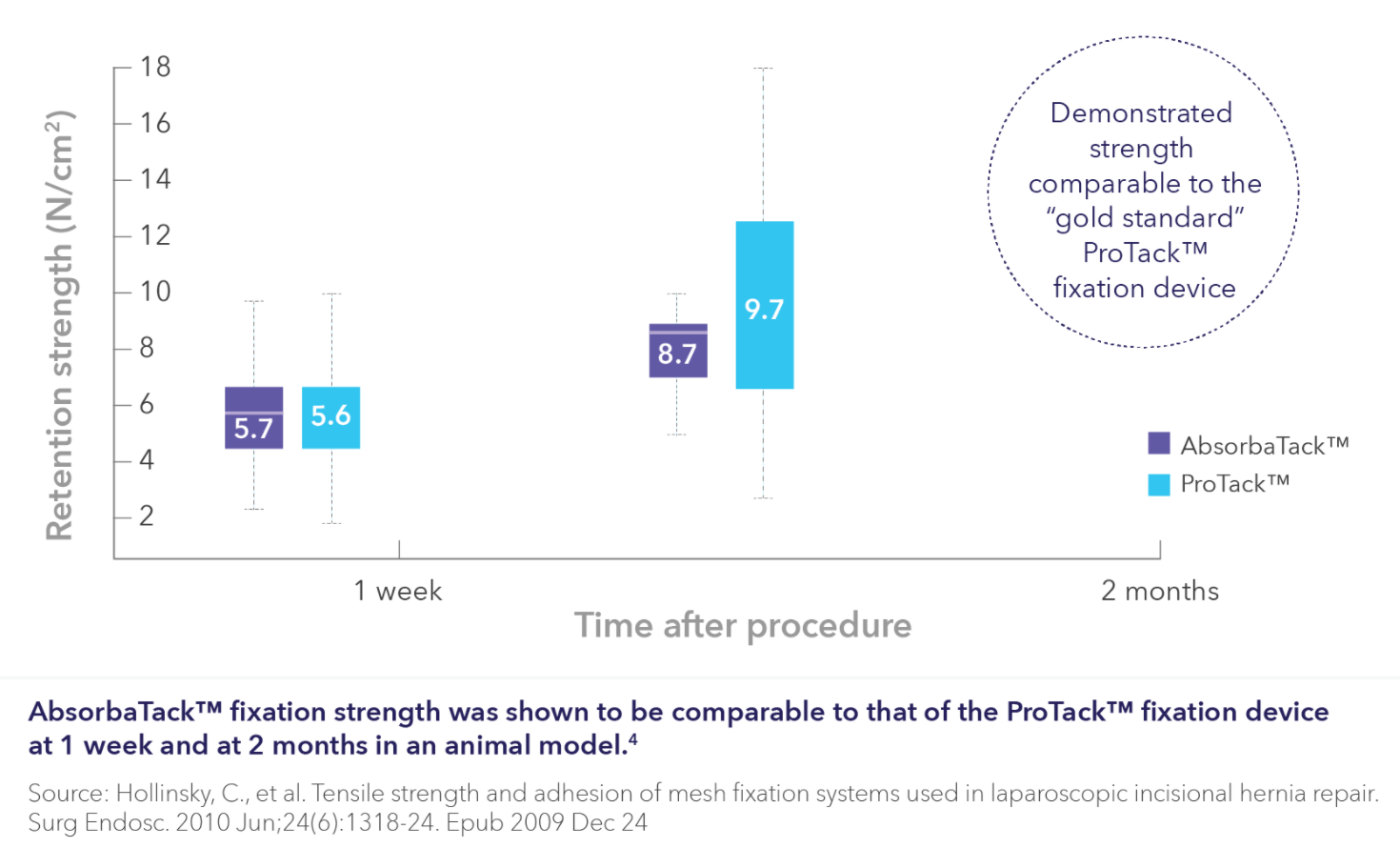
ΩCompared to competitive permanent fxation tackers Capsure™* and Medtronic ProTack™ devices.
††Based on surgeon feedback.
‡‡Compared to competitive and Medtronic absorbable and permanent fxation tackers.
References:
1. Based on memorandum #RE00450435 Rev A, MaxTack™ frst motorized fxation device memo report. March 2023.
2. Based on internal report #RE00435620, MaxTack™ device reliability demonstration report. January 2023.
3. Based on internal report #RE00406203, MaxTack™ device blue overmold trigger button testing and reliability. May 2022.
4. Based on internal report #RE00437048, MaxTack™ device fxation strength claims. January 2023.
5. Based on internal report #RE00422041 Rev A, Effects of instrument design on body posture with repetitive motions of a lap ventral hernia repair surgeon protocol. October 2022.
6. Based on internal report #RE00422048, Effects of instrument design on body posture with repetitive motions of lap ventral hernia repair surgeon report. August 2022.
7. The Hernia Surgery Group. International guidelines for groin hernia management. January 2018.
©2024 Medtronic. Medtronic, Medtronic logo, and Engineering the extraordinary are trademarks of Medtronic. ™* Third-party brands are trademarks of their respective owners. All other brands are trademarks of a Medtronic company. 10/2023-US-HR-2300113 – [WF#8914114]
Operational Headquarters 710 Medtronic Parkway, Minneapolis, MN 55432-5640 USA
©2025 Medtronic