PRODIGY STUDY
PRediction of Opioid-induced respiratory Depression In patients monitored by capnoGraphY
PRODIGY is a Medtronic-sponsored, prospective, multi-center, international cohort study to identify patients at risk of opioid-induced respiratory depression (OIRD), a potentially life-threatening form of respiratory compromise. The PRODIGY study found a 46 percent incidence of respiratory depression in patients on the GCF receiving parenteral opioids.1
The secondary PRODIGY economics publication concludes that continuous respiratory monitoring has a high chance of being cost-effective based on the hypothetical median-sized hospital model2:
- $535,531 annual hospital costs reduction: Continuously monitoring SpO2 and etCO2 on patients at high risk for respiratory depression is projected to reduce annual hospital costs
- 103 days saved in cumulative LoS: cumulative patient length of stay is projected to be reduced by 103 days†
- Break-even point at 1.5 percent reduction in respiratory depression: break-even investment point is achieved when reducing respiratory depression by 1.5 percent
View an infographic on the highlights of the secondary PRODIGY economics publication.
†This assumes a 20 percent respiratory depression reduction and an annual general care floor volume of 2,447 patients receiving opioids per median-sized hospital. 90% of surgical patients and 45% of medical patients on U.S. general care floors receive opioids. Continuous pulse oximetry and capnography device pricing assumptions used list pricing for the following: a Capnostream™ 35 portable respiratory monitor ($4300) prorated over 7 years; a Microstream™ capnography filter line ($14.50), and a disposable Nellcor™ pulse oximetry sensor ($8.50), resulting in $52.73 in device costs per continuously monitored patient stay on a medical surgical floor. For intermittent pulse oximetry monitoring, device pricing consisted of a multiparameter monitor ($3000) prorated over 7 years and a reusable pulse oximetry sensor ($65), resulting in $0.68 in device costs per patient stay. Additional information on pricing and assumptions are available in the study publication.
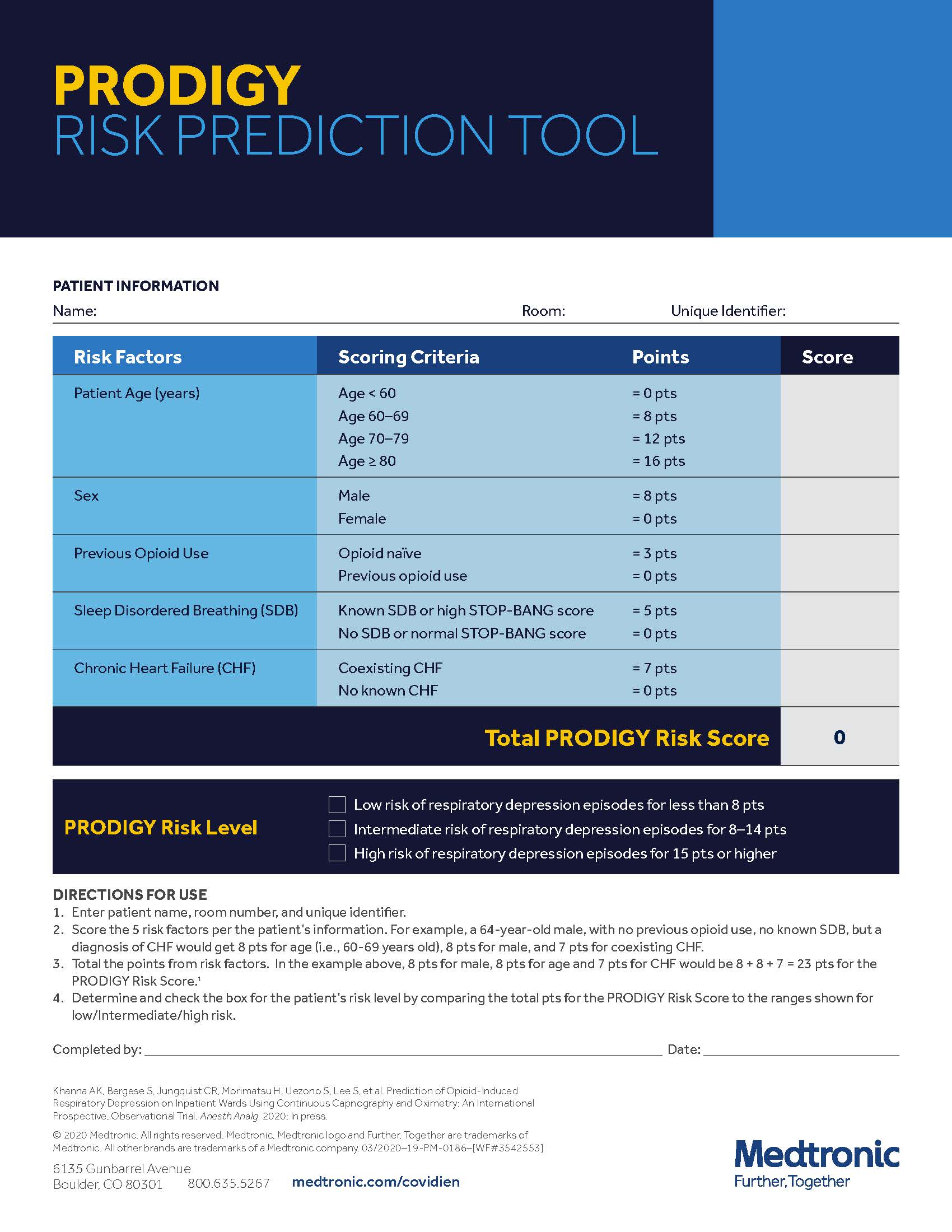
PRODIGY RISK PREDICTION TOOL INTERACTIVE PDF
Until now, no standardized tool has been available to assess risk of developing respiratory depression (RD), a form of respiratory compromise. The PRODIGY Risk Prediction Tool is an easy-to-use tool that helps identify which patients would benefit most from continuous monitoring.
Sign up to receive more information about the PRODIGY study:
The Microstream™ capnography and Nellcor™ monitoring systems should not be used as the sole basis for diagnosis or therapy and are intended only as an adjunct in patient assessment.
1. Khanna AK, Bergese S, Jungquist CR, et al. Prediction of opioid-induced respiratory depression on inpatient wards using continuous capnography and oximetry: an international prospective, observational trial. Anesth Analg. Vol. 131(4). October 2020.
2. Khanna A, Junquist C, Buhre W, Soto R, di Piazza F, Saager L. Modeling the Cost Savings of Continuous Pulse Oximetry and Capnography Monitoring of United States General Care Floor Patients Receiving Opioids Based on the PRODIGY Trial. Adv Ther. 2021 May 24:1–15. doi: 10.1007/s12325-021-01779-7.