WHAT IS CCHD IN NEWBORNS?
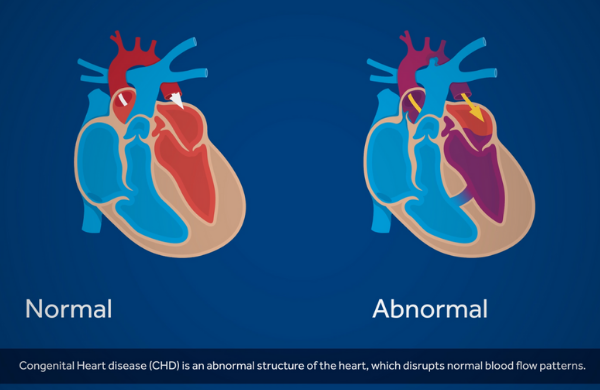
Congenital Heart Disease (CHD) is an abnormal structure of the heart, which disrupts normal blood flow patterns. It is the most common birth defect. Critical Congenital Heart Disease, or CCHD, is a subset of CHD which can bring a significant risk of disability or death if not diagnosed in time 1.
In the United Kingdom, 24.7 out of 10,000 babies are born every year with CCHD 2. These babies are at significant risk if this condition goes undiagnosed.
PULSE OXIMETRY FOR CCHD SCREENING
CCHD can be detected using pulse oximetry tests to measure the newborn’s oxygen saturation. Pulse Oximetry Screening (POS) is a non-invasive, painless and low-cost test that aids in identifying CCHD. By placing sensors on the hand and foot, oxygen saturation can be measured at both sites, with the levels and difference indicating the likelihood of CCHD 3. POS is a highly specific and moderately sensitive test for diagnosing CCHD with a very low false-positive rate 4.
In 2013 a Cairo University Children Hospital review showed that per day 2,000 children were seen in various specialty clinics. Anually, 1,000 paediatric catheterisations and over 6,000 echocardiograms were performed, out of which 30% were cyanotic CHD patients 5. Prior to the COVID-19 pandemic, Egypt created a successful care network for around 20,000 babies born with CHD each year 5. This network consists of a Cairo based central, specialised CHD hospital as well as referral networks of specialised paediatric centers around the country.
Would you like to know more about CCHD screening?
WHY USE NELLCOR™ PULSE OXIMETRY FOR CCHD SCREENING
The Nellcor™ pulse oximeter is cleared for motion-tolerance with labelling for Use in Screening of Newborns for CCHD and it confirms the reliable performance of Nellcor™ pulse oximeters during motion. Nellcor™ pulse oximetry with Oximax™ technology meets key challenges in newborns: Speed 6,7, Accuracy 8, Motion 9, Perfusion 10 and Sensitivity 11.
Discover the five Medtronic solutions for CCHD screening with Pulse Oximetry:
I. TECHNOLOGICAL EXCELLENCE:
Nellcor™ pulse oximeters and Nellcor™ sensors are designed to meet these CCHD screening requirements and overcome the following challenges with newborns 6-11:

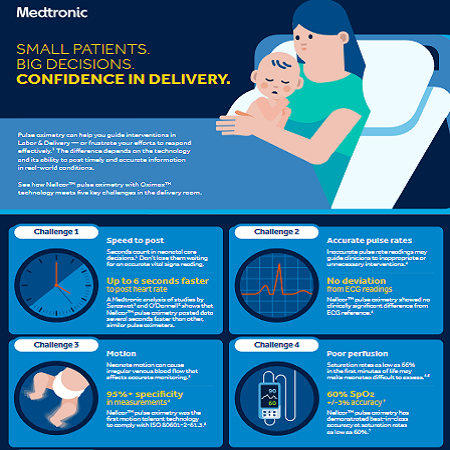
SPEED TO POST
SOLUTION
Nellcor™ pulse oximetry posts data several seconds faster than other, similar pulse
oximeters 6,7.

POOR PERFUSION
SOLUTION
Nellcor™ pulse oximetry has demonstrated accuracy at saturation rates as low as 60%10.
MOTION
SOLUTION
Nellcor™ pulse oximetry is cleared as motion tolerant and is in compliance with standard 80601‑2‑61:2011.8 9.
ACCURATE PULSE READINGS
SOLUTION
Nellcor™ pulse oximetry shows no clinically significant difference from ECG reference 8.
SKIN SENSITIVITY
SOLUTION
Nellcor™ non‑adhesive sensor is made of soft, pliable, low-profile foam material that fastens securely to itself with hook and loop fasterner instead of adhesive tape, to help protect sensitive skin 11.
II. ECONOMIC FLEXIBILITY:
Nellcor™ sensors provide flexibility for every budget as they are available in a range of solutions: disposable, semi-disposable and non-adhesive.
III. CLINICAL SUPPORT:
We provide you access to a wealth of clinical evidence supporting CCHD screening strategies, including protocols from the United States and Europe, as well as key findings from 21 studies carried out on almost half a million patients.
IV. TRAINING & EDUCATION:
Our comprehensive educational portfolio delivers resources in multiple formats including online courses, live training events and access to Local Support Teams to achieve the best from Nellcor™.
V. MOBILE SCREENING & AUTOMATION:
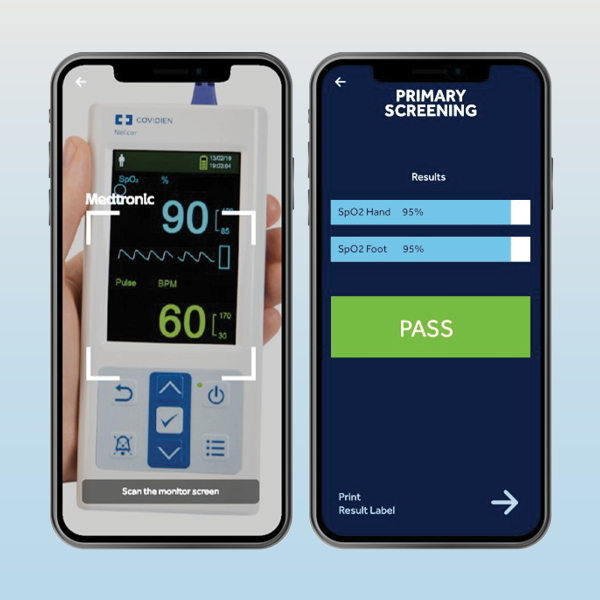
And to make your CCHD screening test easier, smarter and more convenient, download our NEW mobile screening & automation application, the Nellcor™ CCHD Screening App, which provides instant feedback by inputting the recorded values manually, or even by scanning the screen of your pulse oximeter.
SMALL PATIENTS – BIG DECISIONS
Discover in this video the extensive clinical and medical portfolio Medtronic offers to support you in screening babies for CCHD.
Would you like to receive more information on how Medtronic can partner with you to early detect CCHD and improve your patients' safety?
Please complete this form and we will contact you as soon as possible.
- * Pulse oximetry screening for critical congenital heart defects: a European consensus Statement.The Lancet Child and Adolescent Health 2017; published online Aug 30. http://dx.doi.org/10.1016/S2352-4642(17)30066-4
- ** Motion tolerant monitors include Nellcor™ PM100N and PM1000N SpO2 monitoring systems.
- (AHA) Congenital Cardiovascular Defects: Current Knowledge: A Scientific Statement from the American Heart Association Council on Cardiovascular Disease in the Young. Circulation, volume 115, June 12, 2007: 2995-3014.
- Bakker MK, Bergman JEH, Krikov S, et al. Prenatal diagnosis and prevalence of critical congenital heart defects: an international retrospective cohort study. BMJ Open 2019;9:e028139. doi:10.1136/bmjopen-2018-028139
- Kemper AR, Mahle WT, Martin GR, Cooley WC, Kumar P, Morrow WR, Kelm K, Pearson GD, Glidewell J, Grosse SD, Howell RR. Strategies for implementing screening for critical congenital heart disease. Pediatrics. 2011 Nov;128(5):e1259-67. doi: 10.1542/peds.2011-1317. Epub 2011 Oct 10. PMID: 21987707.
- Riede FT, Wörner C, Dähnert I, Möckel A, Kostelka M, Schneider P. Effectiveness of neonatal pulse oximetry screening for detection of critical congenital heart disease in daily clinical routine--results from a prospective multicenter study. Eur J Pediatr. 2010 Aug;169(8):975-81. doi: 10.1007/s00431-010-1160-4. Epub 2010 Mar 1. PMID: 20195633; PMCID: PMC2890074.
- El-Saiedi, S.A., Haeffele, C., Hanna, B.M., Lui, G.K. The Hidden Victims of the COVID-19 Pandemic: Congenital Heart Disease Patients. JACC Case Rep. 2020 Jul 15;2(9):1411-1413. doi: 10.1016/j.jaccas.2020.05.081
- Saraswat A, Simionato LK, Dawson JA, et al. Determining the best method for nellcor pulse oximeter sensor application in neonates. Acta Paediatr. 2012;101(5):484‑487. doi: 10.1111/j.1651‑2227.2011.02571.
- O’Donnell CP, Kamlin CO, Davis PG, Morley CJ. Obtaining pulse oximetry data in neonates: a randomized crossover study of sensor application techniques. Arch Dis Child Fetal Neonatal Ed. 2005;90:F84‑F85. doi: 10.1136/adc.2004.058925.
- Rabi Y, Dawson JA. Oxygen therapy and oximetry in the delivery room. Semin Fetal Neonatal Med. 2013;18(6):330‑5. doi: 10.1016/j.siny.2013. 08. 007.
- RE10096395-Report, Clinical, Motion, Connery OEM Module including the clinical evaluations of Connery 3.1 board with N-600x and MaxA and MaxFast sensors.
- System Accuracy and Ranges in Instructions for Use (PN: 10134064). VERIFICATION TEST REPORT, BASIC PERFORMANCE, OXICABLE (PN: 10148583)( PMC10GE360N and PMC10GE120N). Verification & Validation Summary Report (PN: RE00112546)(EXT). Insert, Pointer to GE Instructions for Use (PN: PT00062986).
- 10077105 -SoftCare Sensor Peer Review includes verification that the patient contact material is a 1/32’’ PVC closed cell, polyvinyl chloride foam material, 3M 9777L. The Sensor Face drawings (064923 REV B (SC-A), 066042 REV C (SC-PR), 066819 REV D (SC-NEO) and ‘where used’ reports from Agile demonstrate that the patient contact surface is specified in 901813 REV A. A BOM report from Agile is included for each sensor face to coordinate between the face assembly drawing and the patient contact material. 10077105 -SoftCare Sensor Peer Review includes verification that the sensor is secured to the patient using an integral Velcro closure, a Velcro cable wrap is included for anchoring the cable, and that the sensor does include additional adhesives or sticky rings to extend the use of the sensor. The peer review references the following IFU process instructions (see attachments to RE10077105 for details):
IFU: 10035575 rev C (SC-A/SC-A-I), 10056240 rev B (SC-NEO/SC-NEO-I), 10035647 rev B (SC-PR/SC-PR-I) – for attachment methodPI065868 rev L: Process Instruction, SoftCare Sensor – verification of package content specificationProduct samples: SC-A: lot # 0123072, SC-NEO: lot #8144035, and SC-PR: lot #8032039 – verification of package contents.